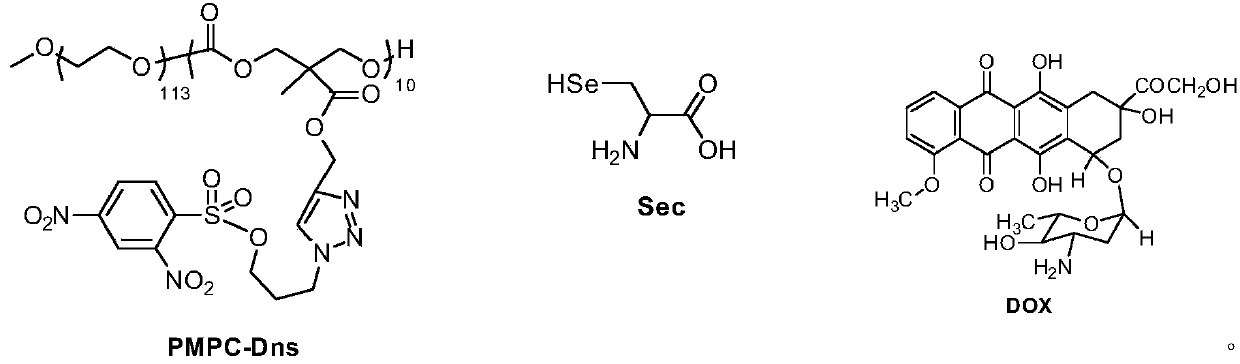
A new method for detecting selenocysteine in living body
A technology of selenocysteine and living body, which is applied in the synthesis of polycarbonate micelles and the detection of selenocysteine, achieving the effect of low cost and easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
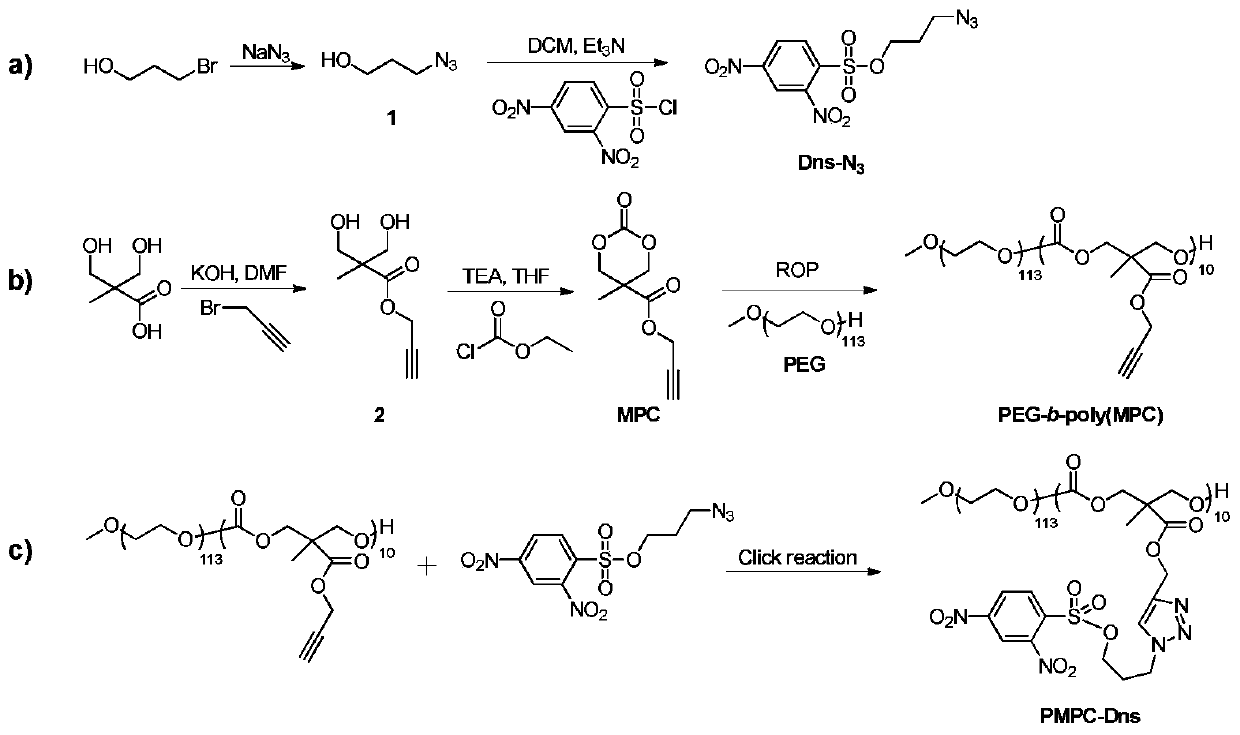
[0015] Please refer to the accompanying drawings for the reaction route for synthesizing the dinitrobenzenesulfonyl-modified biodegradable polycarbonate micelle compound provided by the present invention.
[0016] Below in conjunction with concrete preparation example, the present invention will be further described:
[0017] Synthesis of PMPC-Dns micelles
[0018] Synthesis of 3-Azido-1-propanol
[0019] Into a 50mL reaction tube, add 1.02g 3-bromopropanol, 0.95g sodium azide, then add 10mL distilled water, react at 80°C for 18h, stop heating, cool to room temperature, add ethyl acetate for extraction, take the upper layer, Then washed with saline solution, dried over anhydrous magnesium sulfate, filtered, the filtrate was collected, and the solvent was removed to obtain colorless 3-azido-1-propanol with a yield of 76.5%.
[0020] Synthesis of propyl 2,4-dinitrobenzenesulfonate (Dns-N 3 )
[0021] Into a 50mL round bottom flask, add 0.57g 3-azidopropanol, 1.56mL triethyla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com