Alisma 3-hydroxy-3-methyl glutaric acyl coenzyme A reductase antibody preparation and detection method
A technology of methylglutaryl coenzyme and reductase, applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
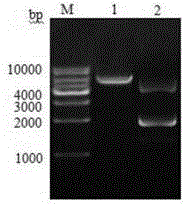
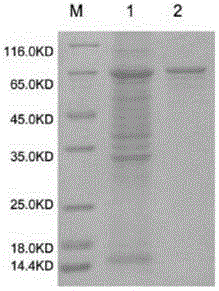
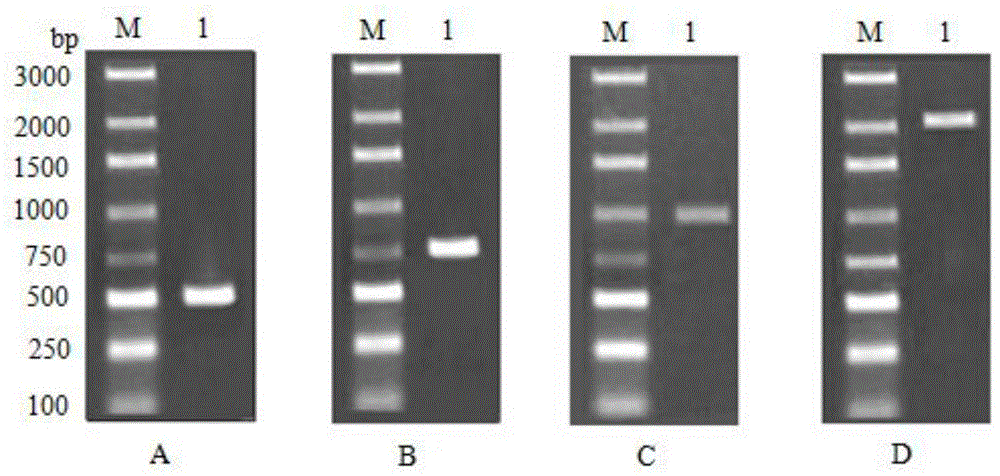
[0065] Using homologous cloning method and RACE technology to clone the full-length cDNA of Alisma 3-hydroxy-3-methylglutaryl-CoA reductase gene, polyclonal antibody preparation method, prokaryotic expression and purification of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene Diacyl-CoA reductase protein was used to prepare polyclonal antibody in immunized rabbits.
[0066] The full-length cDNA of Alisma 3-hydroxy-3-methylglutaryl-CoA reductase gene was cloned by using the homologous cloning method and RACE technology above, and the full-length AoHMGR was cloned on the basis of the conserved region fragment of the AoHMGR gene obtained earlier. Alisma RNA was extracted by the kit method, cDNA was obtained by reverse transcription with Oligo dT18 primers, the fragment cloning of the conserved region of the AoHMGR gene was carried out, and RACE and full-length cDNA amplification were performed.
[0067] The methods for cloning the conserved region of the above-mentioned AoHM...
Embodiment 2
[0074]Purification is to prepare an antigen affinity purification column by coupling the protein with agarose medium, mix the obtained antiserum with PBS in equal amounts, and slowly load the sample, and elute with glycine elution buffer after the antigen and antibody are combined to obtain The desired purified antibody was immediately dialyzed in PBS at 4°C overnight, and the purity, concentration and titer were determined the next day.
[0075] The method for determining the concentration of the purified antibody comprises the following steps:
[0076] (1) Preparation of BCA working solution: According to the number of samples, prepare an appropriate amount of BCA working solution according to 50 volumes of BCA reagent A plus 1 volume of BCA reagent B (50:1), and mix well.
[0077] (2) Take an appropriate amount of BSA protein standard as needed, add deionized water to dilute to 1 mg / mL (stock solution 5 mg / mL), and mix well.
[0078] Note: In principle, whatever solution t...
Embodiment 3
[0090] Take the purified 3-hydroxy-3-methylglutaryl-CoA reductase protein, and immunize 2-2.5Kg New Zealand white rabbits by subcutaneous injection, 400μg / time, once every 2-3 weeks, and immunize 4 times in total ;Blood testing, determine the titer of the antiserum against the protein by indirect ELISA method, wait for the titer to be greater than 1:50,000 for final blood collection to prepare antiserum, and purify polyclonal antibodies.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com