Ferritic stainless steel sheet having excellent brazability, heat exchanger, ferritic stainless steel sheet for heat exchangers, ferritic stainless steel, ferritic stainless steel for members of fuel supply systems, and member of fuel supply system
一种不锈钢板、热交换器的技术,应用在热交换器固定、热交换设备、具有防腐蚀措施的燃料喷射装置等方向,能够解决未提出耐腐蚀性和钎焊性不锈钢等问题,达到耐腐蚀性优良、优良强度、钎焊性优良的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 Embodiment approach
[0066] The object of this embodiment is a ferritic stainless steel plate used as a raw material of a member obtained by brazing using a nickel brazing filler metal or a copper brazing filler metal. Brazing is performed at 950-1200°C in vacuum or in a hydrogen atmosphere. At this time, argon gas, nitrogen gas, or the like may be used in combination as a controlled atmosphere or for substitution. In brazing, the brazing material wets the master batch (raw material) and fills gaps, thereby joining the master batch. When an oxide film exists on the surface of the raw material, wettability becomes difficult and brazeability is hindered.
[0067] A Cr (Fe, Cr)-rich oxide film is formed on the surface of the stainless steel plate, thereby showing excellent corrosion resistance. In order to ensure wettability, it is necessary to remove this coating, and to restore the coating, it is brazed under conditions of a vacuum degree or a low dew point. Specifically, the brazing of stainles...
no. 2 Embodiment approach
[0146] First, brazing properties will be described. This embodiment is aimed at the brazing of members made of stainless steel using nickel brazing filler metal or copper brazing filler metal. Brazing wets members by brazing and fills and joins gaps between members. When an oxide film exists on the surface of the stainless steel constituting the member to be brazed, the member becomes difficult to wet and brazeability is hindered.
[0147] A Cr (Fe, Cr)-rich oxide film (passive film) is formed on the surface of stainless steel, thereby showing excellent corrosion resistance. In order to ensure the wettability of stainless steel, it is necessary to reduce and remove this oxide film during brazing. When brazing a stainless steel plate, it is usually kept at the brazing temperature for about 10 to 30 minutes. How to reduce the film formed on the surface of the stainless steel within this limited time has a great influence on the brazeability.
[0148] In view of this backgrou...
Embodiment 1
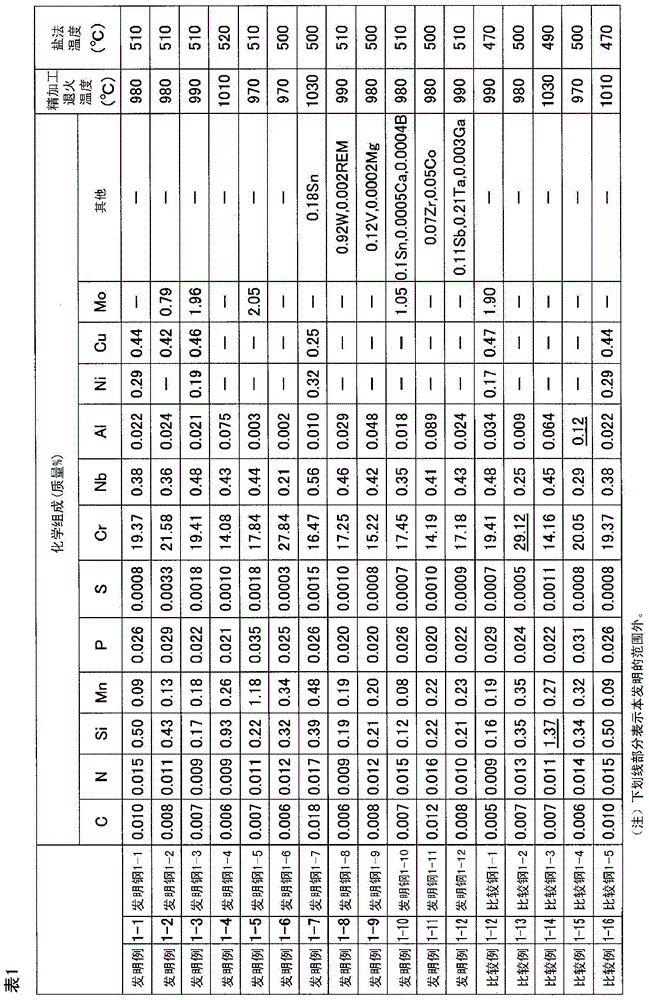
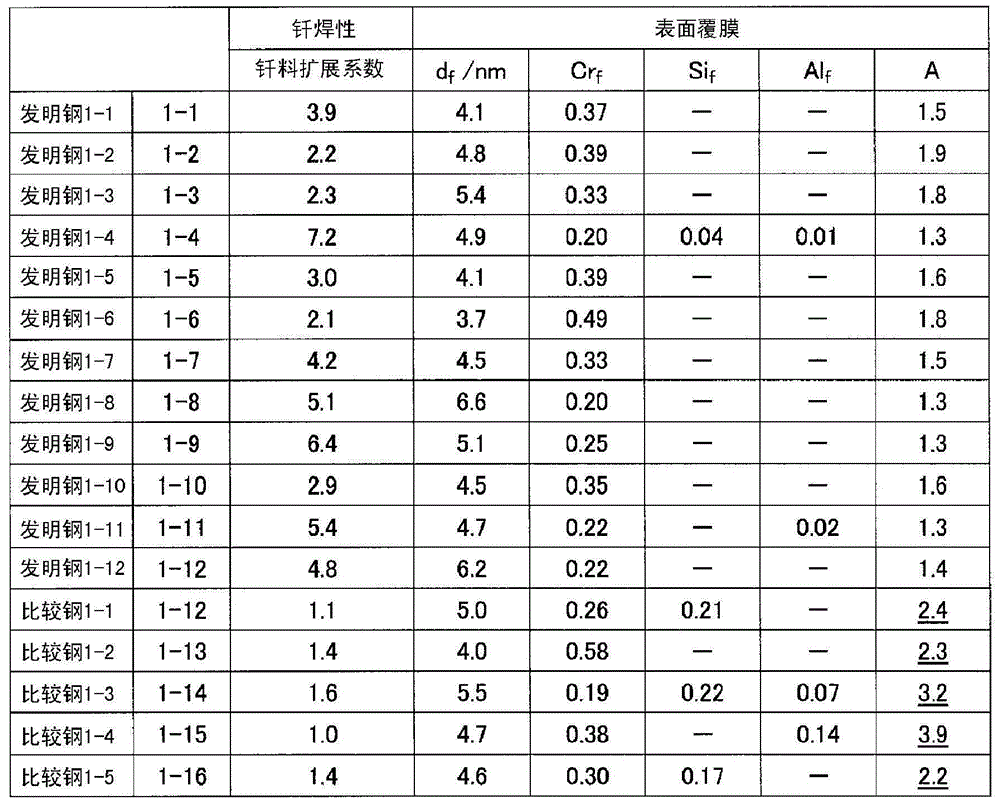
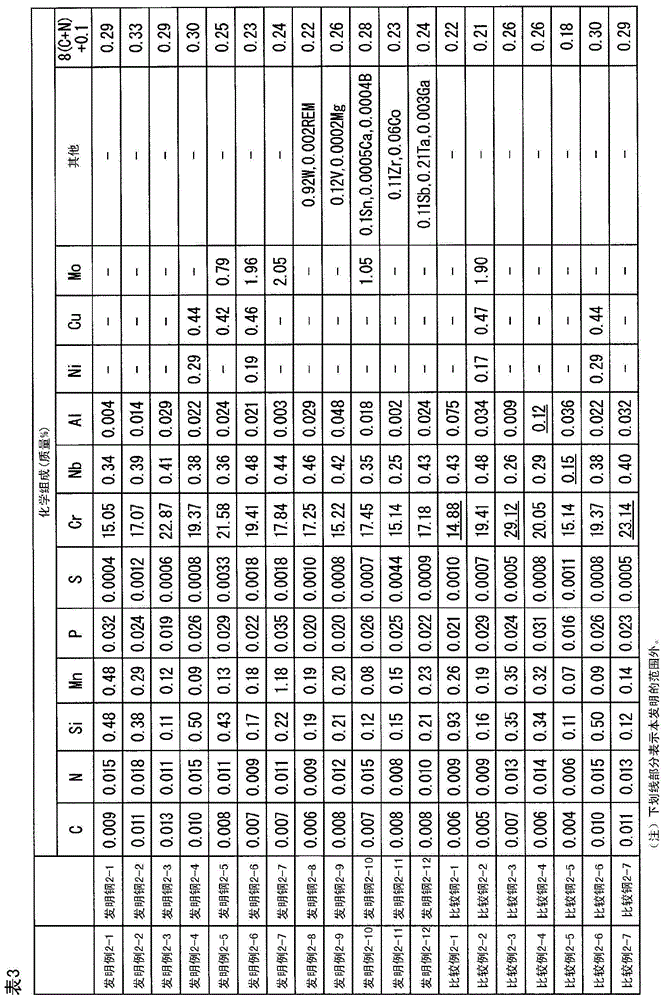
[0236] 30 kg of molten steel having the chemical composition shown in Table 1 was melted in a vacuum melting furnace to produce a 17 kg flat steel ingot. Next, the steel ingot was hot-rolled at a heating temperature of 1200° C. to a thickness of 4.5 mm. The hot-rolled sheet was annealed at 950° C., and then the scale was removed by aluminum shot blasting, and the hot-rolled sheet was cold-rolled to a sheet thickness of 1 mm. After that, finishing annealing is performed, and scale is removed by salt method and immersion in nitric acid-hydrofluoric acid.
[0237] The finishing annealing temperature was the temperature shown in Table 1, and the holding time was 1 minute.
[0238] As the salt method, a method of heating a commercially available descaling alkali salt mainly composed of NaOH and immersing the steel sheet in the alkali salt was used. The heating temperature was set to the temperature shown in Table 1 and the immersion time was 5 seconds.
[0239] In the impregnatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| longitudinal tensile yield strength | aaaaa | aaaaa |
| longitudinal tensile yield strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com