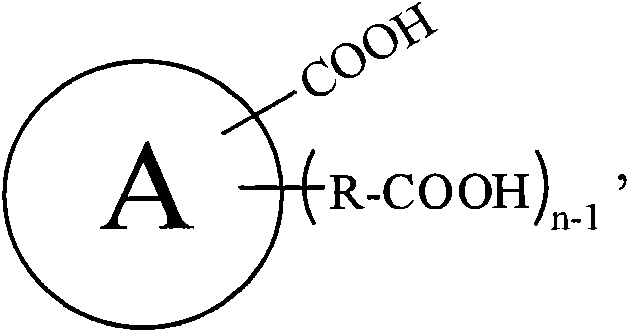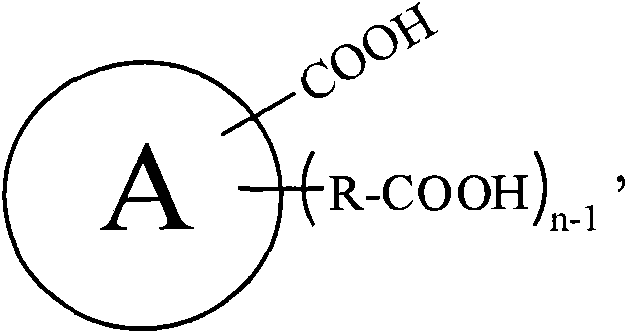Preparation methods of nitrile and corresponding amine
A manufacturing method and amide technology, which are applied in chemical instruments and methods, dehydration preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve the problems of pollution, high energy consumption, environmental pressure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0121] The following examples are used to further illustrate the present invention in detail, but the present invention is not limited to these examples.
[0122] Example of preparation of amide intermediate
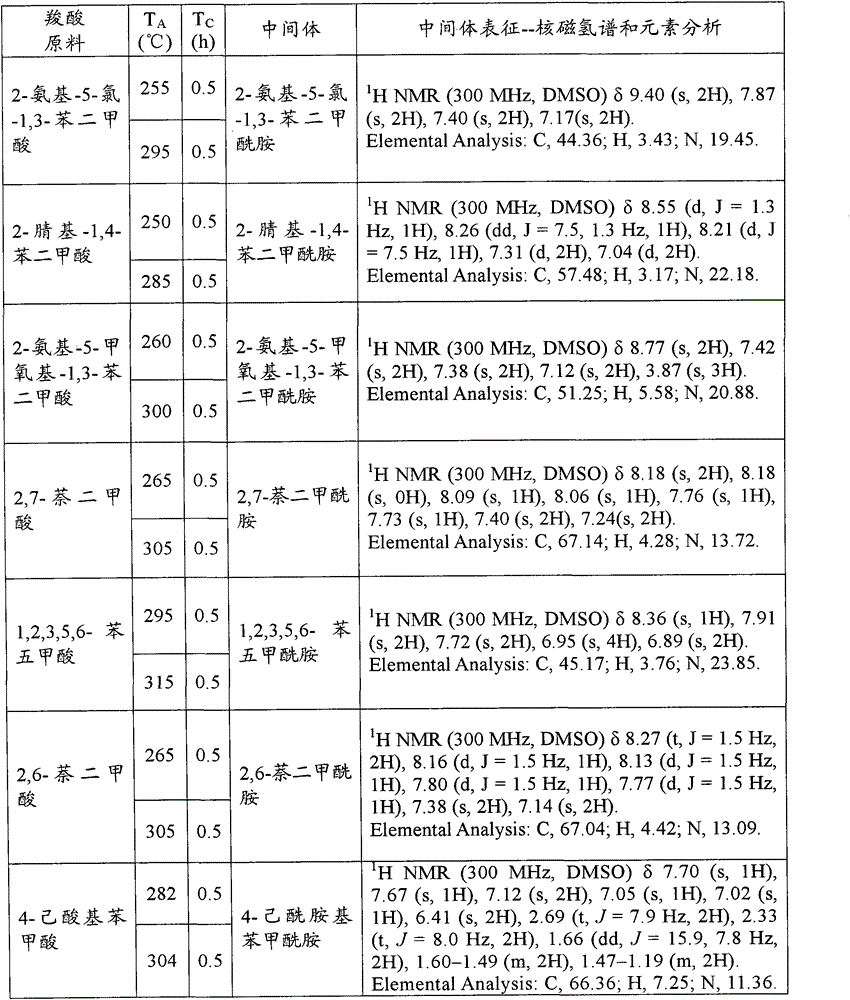
[0123] Add 500g of carboxylic acid raw material (chemically pure) into the 1L open reactor, turn on the stirring (600r / min), and continuously inject ammonia gas (chemically pure, with a water content of 5.1wt%) into the carboxylic acid raw material from the bottom of the reactor. 100g / min). Make the reaction at reaction temperature T A Proceed to T C After hours, stop the ammonia gas. Sampling the contents of the reaction kettle, do hydrogen nuclear magnetic spectrum and elemental analysis to characterize the amide intermediate. The specific reaction conditions and characterization results are shown in Table A-1, Table A-2, Table A-3 and Table A-4 below. These characterization results indicate that the obtained amide intermediate has extremely high purity (above 99%).
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com