Method for synthesizing biphenyl by stille reaction of organotin compound recycling
A compound and organotin technology, applied in the field of organotin recycling application, can solve the problem of low utilization rate of organotin, and achieve the effect of optimizing synthesis process conditions and effective separation and utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
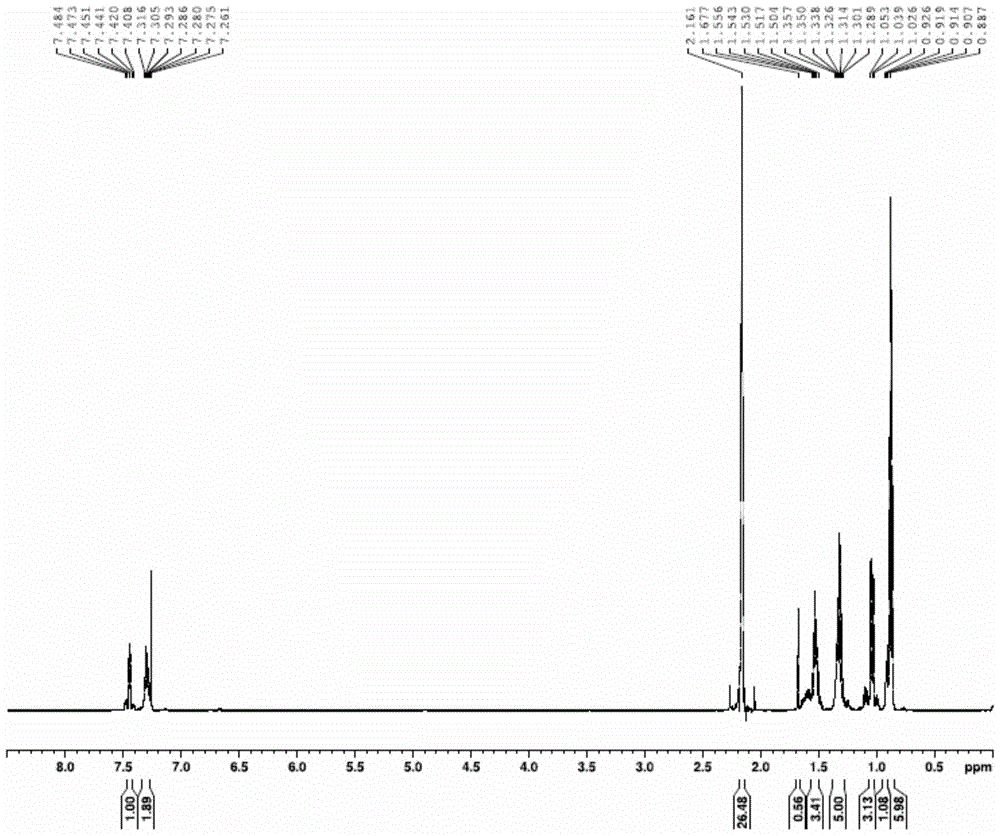
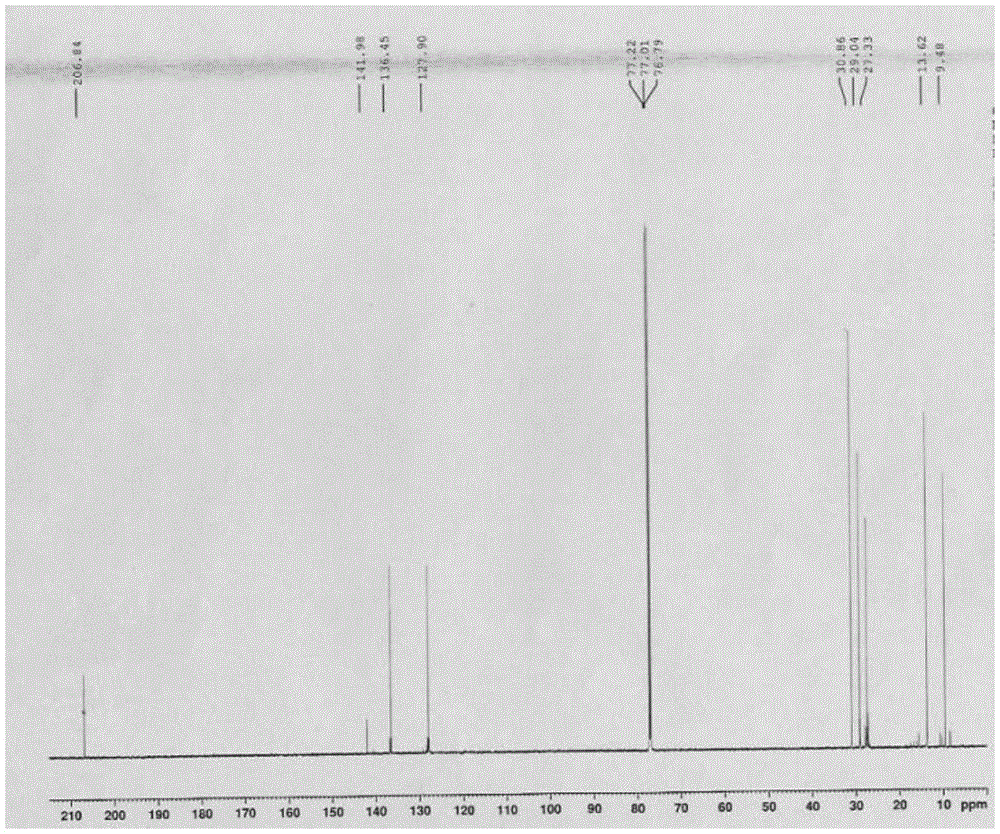
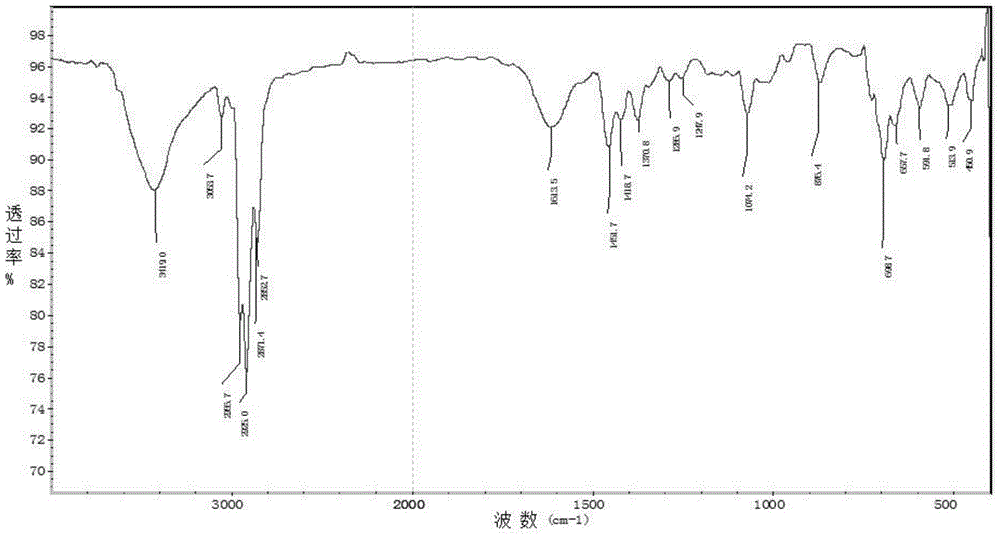
[0035] Specific embodiment one: the method for synthesizing biphenyl by Stille reaction of organotin compound recycling in this embodiment is implemented according to the following steps:
[0036] 1. Add metal magnesium chips and bromobenzene into a three-necked flask with a constant pressure dropping funnel and a drying tube, replace with nitrogen three times, heat and reflux for 1.5-2.5 hours, cool to room temperature, place in an ice-water bath, and add dropwise Tributyltin chloride tetrahydrofuran mixed solution, gray-white precipitate was obtained, and then continued to reflux for 12-14 hours, then added saturated ammonium chloride solution, extracted with ether, dried with anhydrous magnesium sulfate, removed impurities, and obtained light yellow liquid by suction filtration. After vacuum distillation, the cuts were collected to obtain a colorless solution of tributylphenyltin (M R = 364.13);
[0037]2. Use a syringe to inject halobenzene, tributylphenyl tin, cuprous io...
specific Embodiment approach 2
[0042] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is that in step 1, the material ratio of metal magnesium chips, bromobenzene and tributyltin chloride is 1.0:1.0:1.0-1.1. Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0043] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is that in step one, metal magnesium chips and bromobenzene are added to a three-necked flask with a constant pressure dropping funnel and a drying tube, and nitrogen is replaced three times. Heat to reflux at ~45°C for 1.5~2.5h. Other steps and parameters are the same as those in Embodiment 1 or Embodiment 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com