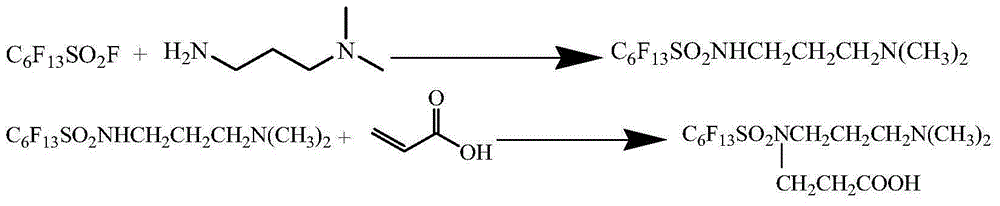
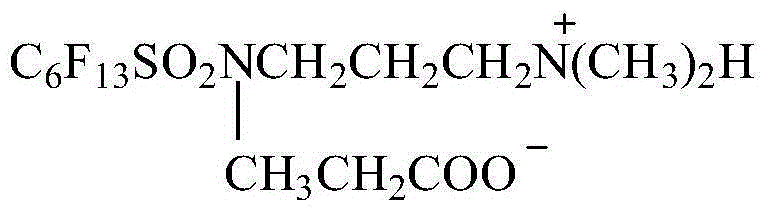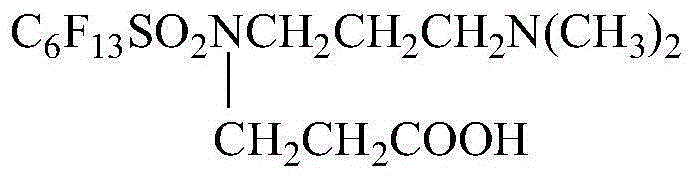Preparation method for surfactant N-carboxyethyl, N-3-dimethylaminopropyl-perfluoro hexyl sulfonamide
A technology of dimethylaminopropyl and sulfonamides, which is applied in the preparation of sulfonamides, chemical instruments and methods, fire prevention equipment, etc., can solve the problems that the products are not purified and affect the performance of the products, and avoid many by-products , reduce volatilization, reduce the effect of self-polymerization reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Synthesis of N-3-dimethylaminopropyl-perfluorohexylsulfonamide
[0032] Add 84g of dimethylaminopropylamine and 600g of toluene into a 1000ml flask, start stirring, add 164g of perfluorohexylsulfonyl fluoride dropwise at room temperature, and the addition is completed within 30 minutes. The reaction was stirred at 30-40°C for 6h. Suction filtration, washing with water 3 times, and drying to obtain a white powder solid with a yield of 99.3% and a melting point of 128-130°C.
[0033] Anhydrous ethanol was recrystallized to obtain a white solid with a yield of 86.3% and a melting point of 129-131°C.
[0034] 1HNMR(600M,CDCl3):δ1.765(t,2H,-CH2-),δ2.3(s,6H,-CH3),δ2.616(t,2H,-CH2-),δ3.490(t ,2H,-CH2-).
[0035] IR(KBr): ν520.20cm-1 has -CF2- flexural vibration absorption peak, 1147.07cm-1 has -CF2- asymmetric stretching vibration peak, and 1193.71cm-1 has S=O strong absorption peak of stretching vibration , 1363.27cm-1 has a strong characteristic absorption peak of SN as...
Embodiment 2
[0036] Example 2 Synthesis of N-3-Dimethylaminopropyl-perfluorohexylsulfonamide
[0037] In a 500ml flask, 21g of dimethylaminopropylamine, 21g of triethylamine and 150g of toluene were added, stirring was started, 82g of perfluorohexylsulfonyl fluoride was added dropwise at room temperature, and the addition was completed in 30 minutes. The reaction was stirred at 30-40°C for 4h. Suction filtration, washing with water 3 times, and drying to obtain a white powder solid with a yield of 97.3% and a melting point of 116-119°C. .
[0038] Recrystallization from methanol gave a white solid with a yield of 76.5% and a melting point of 128-131°C.
Embodiment 3
[0039] Example 3 Synthesis of N-carboxyethyl, N-3-dimethylaminopropyl-perfluorohexylsulfonamide
[0040] 100g of N-3-dimethylaminopropyl-perfluorohexylsulfonamide and 160g of toluene were added to a 1000ml flask, the temperature was raised to 100°C, and 16g of acrylic acid was added dropwise. The reaction was stirred at 100-110°C for 12h. The solvent was evaporated to obtain a pale yellow solid with a yield of 99.8% and a melting point of 143-147°C.
[0041] Anhydrous ethanol was recrystallized to obtain a white solid product with a yield of 80.0% and a melting point of 160-163°C.
[0042] 1HNMR(600M, MeOD): δ2.063(d,2H,-CH2-),δ2.555(d,2H,-CH2-),δ2.847(s,6H,-CH3),δ3.055(d ,2H,-CH2-),δ3.499(d,2H,-CH2-),δ3.651(d,2H,-CH2-).
[0043] 19FNMR(565M, MeOD): δ-82.37(CF3-), δ-112.92(-CF2-SO2-), δ-121.38(-CF2-C-SO2-), δ-122.76(-CF2-CC-SO2- ), δ-123.76(-CF2-CCC-SO2-), δ-127.32(-CF2-CCCC-SO2-).
[0044] IR(KBr): ν557.90cm-1 has -CF2- flexural vibration absorption peak, 1148.69cm-1 has -CF2- asym...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com