Preparation method for solution type triamcinolone acetonide acetate injection
A technology for triamcinolone acetonide acetate and injection, which is applied in the field of preparation of solution-type triamcinolone acetonide acetate injection, can solve the problems of easy generation of particulate matter in the storage process, complicated process, and no solution to the water solubility of the injection, and achieves easy absorption. , the production process is simple, the effect of local irritation is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
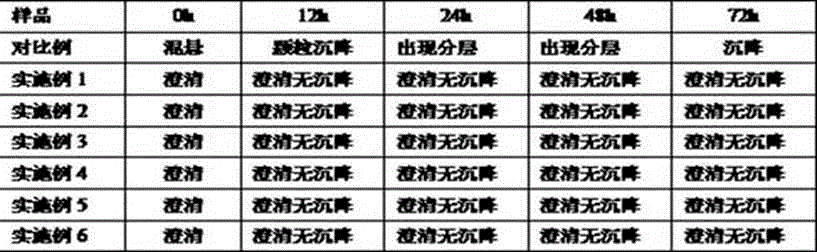
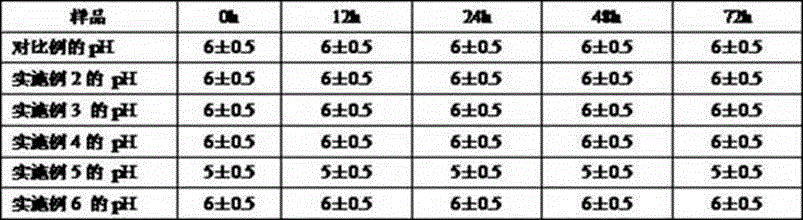
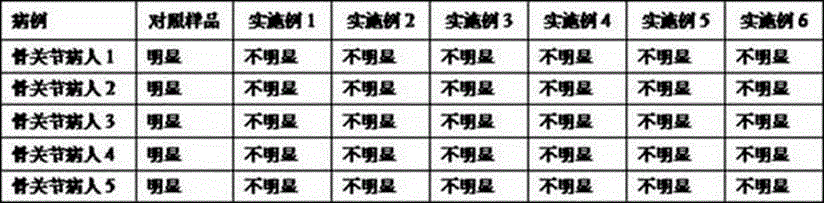
Examples
preparation example Construction
[0032] The present invention provides a kind of preparation method of the injection of solution type triamcinolone acetonide acetate, and described preparation method comprises:
[0033] 1) adding sulfobutyl ether-β-cyclodextrin and triamcinolone acetonide to the organic solvent, stirring and dissolving until clear, and evaporating the organic solvent;
[0034] 2) In the mixture obtained in step 1), add an aqueous solution of sodium hyaluronate, dissolve until clarified, then add dilution water, stir evenly, and prepare sulfobutyl ether-β-cyclodextrin inclusion compound after sterilization Injection.
[0035] The above method, wherein the molar ratio of triamcinolone acetonide acetate and sulfobutyl ether-β-cyclodextrin is 1:1~20, preferably 1:2~10; the ratio of triamcinolone acetonide acetate and water The weight ratio is 1:10-40, preferably 1:20-40, and the molar ratio of triamcinolone acetonide acetate to sodium hyaluronate is 1:1-10, preferably 1:1-5.
[0036] The above ...
Embodiment
[0054] Below in conjunction with specific embodiment, further illustrate the present invention. It should be understood that these examples are only used to illustrate the present invention and are not intended to limit the scope of the present invention. Those skilled in the art can make appropriate modifications and changes to the present invention, and these modifications and changes are all within the scope of the present invention.
[0055] For the experimental methods not indicating specific conditions in the following examples, conventional methods in the art can be used.
[0056] Percentages and parts are by weight unless otherwise indicated. Unless otherwise defined, all professional and scientific terms used herein have the same meanings as commonly understood by those skilled in the art. In addition, any methods and materials similar or equivalent to those described can be applied to the method of the present invention. The preferred implementation methods and ma...
Embodiment 1
[0059] The preparation of embodiment 1 solution type triamcinolone acetonide injection
[0060] Add 1mol of sulfobutyl ether-β-cyclodextrin and 1mol of triamcinolone acetonide acetate to 1L of 75%~100% ethanol, stir and dissolve until clear, and evaporate the organic solvent in a vacuum rotary evaporation; then add 2.5L of 4mol / L sodium hyaluronate aqueous solution, dissolved until clear, then add 2250ml sterilized water for injection, stir evenly, filter and sterilize to prepare an injection containing sulfobutyl ether-β-cyclodextrin inclusion compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com