Ranitidine hydrochloride composition dry suspension for treating gastric ulcer
A technology of ranitidine hydrochloride and dry suspension, applied in the field of medicine, can solve the problems of easy deliquescence, poor stability, discoloration and the like of ranitidine hydrochloride, so as to solve the problems of easy deliquescence, improve stability and simple components Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of ranitidine hydrochloride crystals
[0025] Get ranitidine hydrochloride crude drug, add in the mixed solvent A of water, ethanol, N-methylacetamide whose volume is 8 times of the weight of ranitidine hydrochloride at 30 ℃, water, ethanol, N-methylacetamide Volume ratio is 4:1:1, obtains solution; Apply the constant magnetic field that magnetic field strength is 1.0T then on the horizontal direction of the liquid surface of gained solution, and drop volume is radish hydrochloride in solution under the condition of this constant magnetic field. Acetone 8 times the weight of nitidine; after the dropwise addition, cool down to -10°C, let stand for 2 hours, filter, wash, and dry in vacuum to obtain the ranitidine hydrochloride crystals.
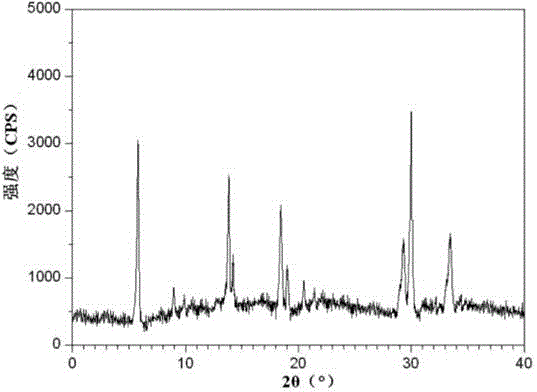
[0026] The X-ray powder diffraction figure that the prepared ranitidine hydrochloride crystal uses Cu-Kα ray measurement to obtain is as follows figure 1 shown.
Embodiment 2
[0027] Example 2: The preparation of ranitidine hydrochloride dry suspension:
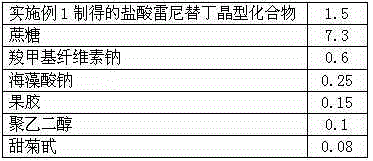
[0028] Prescription: in parts by weight as shown in Table 1
[0029] Table 1 ranitidine hydrochloride composition prescription
[0030]
[0031] Preparation:
[0032] (1) Processing of raw and auxiliary materials: crush ranitidine hydrochloride through a 100-mesh sieve with a pulverizer;
[0033] (2) Weighing: Weigh each raw and auxiliary material according to the process prescription quantity;
[0034] (3) Total mixing: Add all the raw and auxiliary materials in the prescribed amount into the three-dimensional mixer, the mixing speed is 12r / min, and the mixer is turned on for 60 minutes;
[0035] (4) Packaging: Add the granules to the granule packaging machine for packaging, and control the difference in the amount of filling to meet the internal control standards.
Embodiment 3
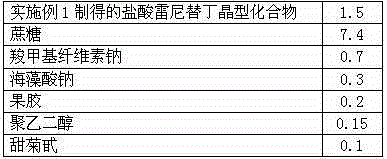
[0036] Example 3: The preparation of ranitidine hydrochloride dry suspension:
[0037] Prescription: in parts by weight as shown in Table 2
[0038] Table 2 ranitidine hydrochloride composition prescription
[0039]
[0040] Preparation:
[0041] (1) Processing of raw and auxiliary materials: crush ranitidine hydrochloride through a 100-mesh sieve with a pulverizer;
[0042] (2) Weighing: Weigh each raw and auxiliary material according to the process prescription quantity;
[0043] (3) Total mixing: Add all the raw and auxiliary materials in the prescribed amount into the three-dimensional mixer, the mixing speed is 12r / min, and the mixer is turned on for 60 minutes;
[0044] (4) Packaging: Add the granules to the granule packaging machine for packaging, and control the difference in the amount of filling to meet the internal control standards.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com