Method for preparing clotrimazole buccal tablets on basis of all-powder direct pressing of modified glucose
A technology of glucose and clotrimazole, applied in the field of medicine, can solve the problems of unstable reproducibility, poor tablet uniformity, poor tableting uniformity, etc., and achieves improved dissolution and uniformity, stable quality, and improved compressibility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
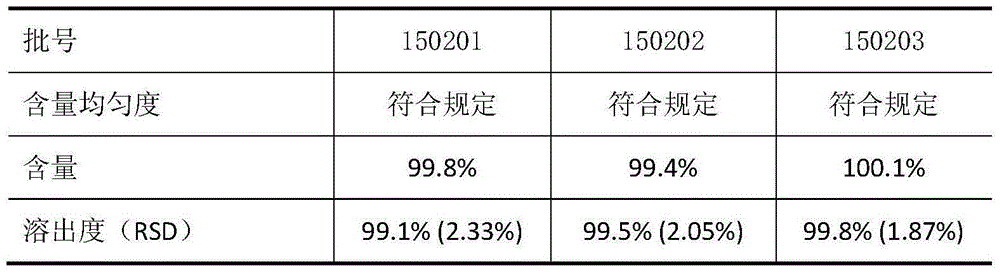
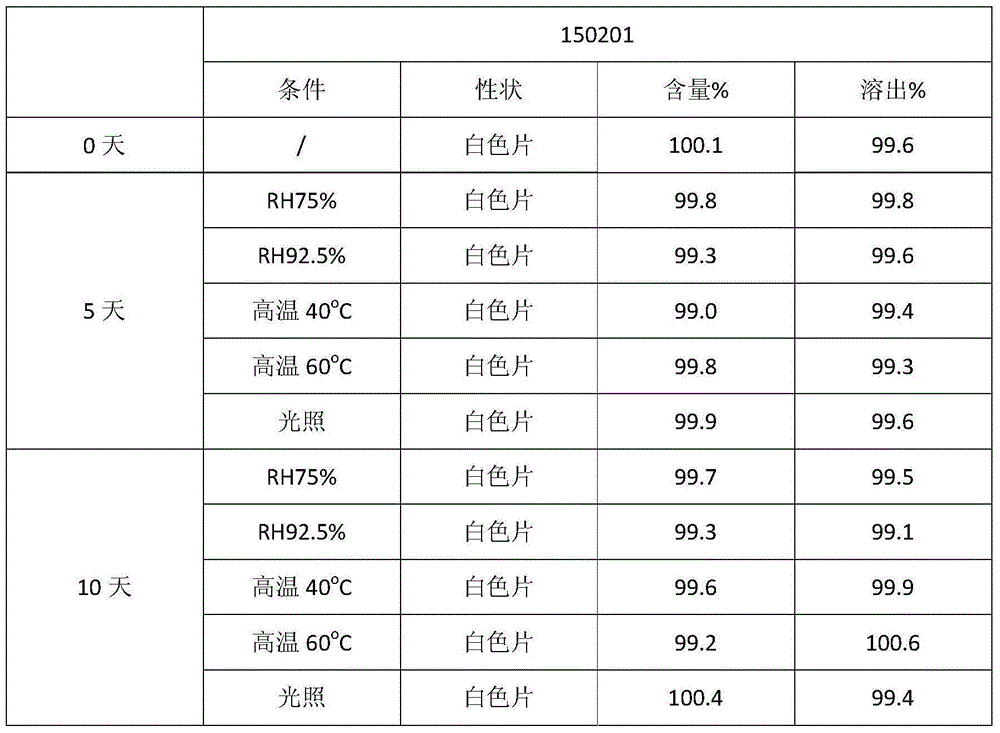
Embodiment 1
[0022] Clotrimazole 10g
[0023] Modified glucose 830g
[0024] Microcrystalline cellulose 50g
[0025] Croscarmellose Sodium 100g
[0026] Povidone K301.3g
[0028] Weigh the prescription amount of clotrimazole and the modified glucose after sizing, and after croscarmellose sodium and microcrystalline cellulose are thoroughly mixed uniformly, add magnesium stearate and mix for 10 minutes. Tableting: Press the DP30A single-punch tablet machine according to the tablet weight range, the rotating speed is 10-12rpm, and the hardness is about 8-15kgf.
Embodiment 2
[0030] Clotrimazole 10g
[0031] Glucose 830g
[0032] Microcrystalline cellulose 50g
[0033] Croscarmellose Sodium 100g
[0034] Povidone K301.3g
[0036] Weigh the prescription amount of clotrimazole and the modified glucose after sizing, and after croscarmellose sodium and microcrystalline cellulose are thoroughly mixed uniformly, add magnesium stearate and mix for 10 minutes. Tableting: Press the DP30A single-punch tablet machine according to the tablet weight range, the rotating speed is 10-12rpm, and the hardness is about 8-15kgf.
Embodiment 3
[0038] Clotrimazole 10g
[0039] Modified glucose 830g
[0040] Microcrystalline cellulose 100g
[0041] Croscarmellose Sodium 50g
[0042] Povidone K302.5g
[0043] Magnesium stearate 10g
[0044] Weigh the prescription amount of clotrimazole and the modified glucose after sizing, and after croscarmellose sodium and microcrystalline cellulose are thoroughly mixed uniformly, add magnesium stearate and mix for 10 minutes. Tableting: Press the DP30A single-punch tablet machine according to the tablet weight range, the rotating speed is 10-12rpm, and the hardness is about 8-15kgf.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com