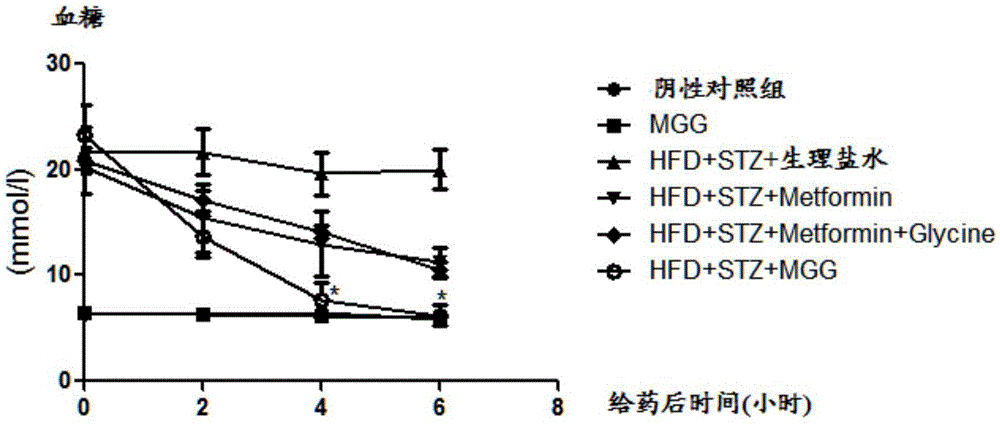
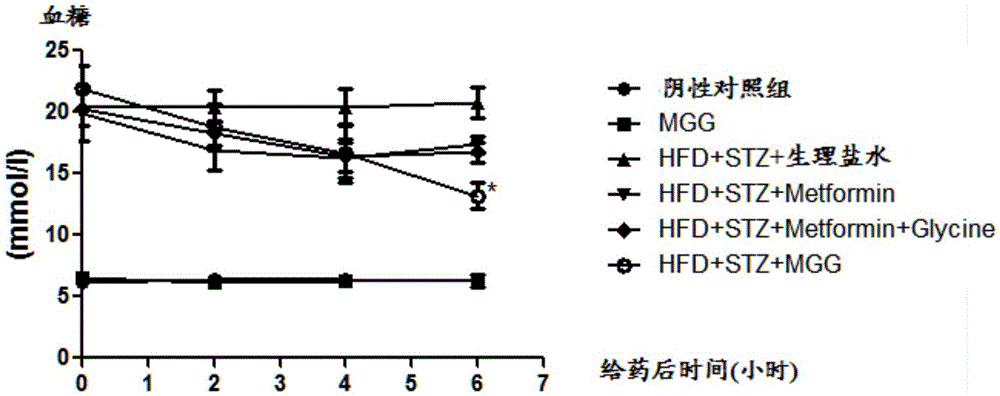
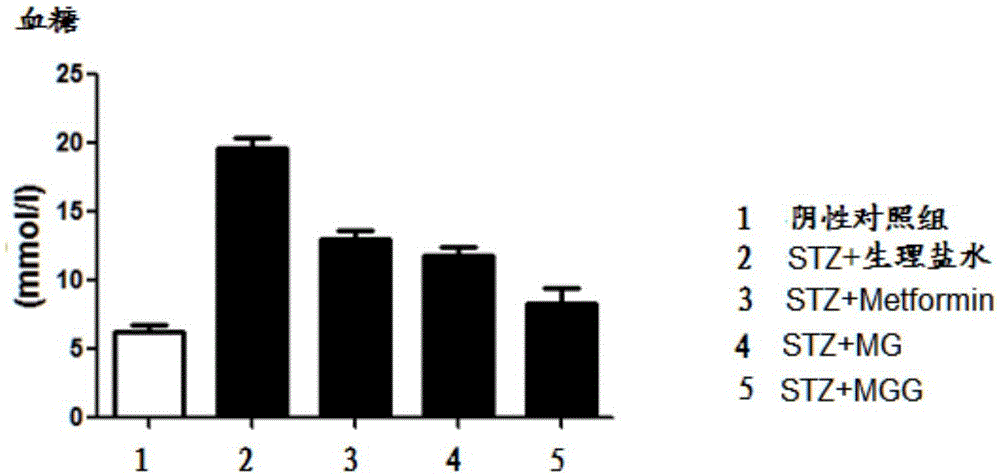
Use of amidation product of glycine and metformin in preparation of drug for treating diabetes
The technology of a diabetes drug, metformin, is applied in the field of preparation of drugs for the treatment of diabetes, which can solve the problems of stably controlling blood sugar and achieve strong hypoglycemic effect and long-term effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of Phthalylglycine (Pht-Gly-OH):
[0024] Put 15.5g (0.105mol) of phthalic anhydride, 7.5g (0.1mol) of glycine, 50mL toluene and 0.8mL triethylamine in a 250mL eggplant-shaped flask, and heat to reflux at 140°C for 8h. After the reaction was completed, cool to room temperature, add 0.6 mL of concentrated hydrochloric acid to the reaction liquid in an ice-water bath, and stir for 30 min. After filtering, the filter cake was washed with water and dried to obtain 20.3 g of white solid with a yield of 99.0%.
[0025] 1HNMR(DMSO-d6,300MHz)δ(ppm):13.21(s,1H,-COOH),7.88-7.90(m,2H,ArH),7.92-7.94(m,2H,ArH),4.32(s,2H ,-CH2-).
[0026] Synthesis of phthaloylglycyl metformin (Pht-Gly-Met):
[0027] Put 4 g (0.024 mol) of metformin hydrochloride (0.024 mol) and 40 mL of sodium hydroxide solution with a concentration of 1 mol / L in a 250 mL eggplant-shaped bottle, and stir at room temperature for 30 min. The reaction solution was spin-dried, and 120 mL of methanol was ad...
Embodiment 2
[0035]Embodiment 2 Synthesis of phthaloylglycylglycyl metformin (Pht-Gly-Gly-Met)
[0036] Take a 250 mL eggplant-shaped bottle, add 2.22 g (0.01 mol) of glycyl metformin hydrochloride (Gly-Met HCl), 150 mL THF, 2.08 mL (0.015 mol) of triethylamine, and stir at room temperature for 3 -4h standby.
[0037] Put 2.04g (0.01mol) of phthalylglycine and 8mL of thionyl chloride in a 50mL eggplant-shaped bottle, stir and reflux at 80°C for 5h, and spin off the excess thionyl chloride to obtain Pale yellow acid chloride for use.
[0038] Add 5.3 g (0.025 mol) of tripotassium phosphate to the above-mentioned THF solution of glycyl metformin, and stir under an ice-water bath. The acid chloride prepared above was dissolved in 20 mL THF, and added dropwise to the metformin solution through a constant pressure dropping funnel. After the dropwise addition, the ice-water bath was removed, and the reaction solution was stirred at room temperature for 2-3 h.
[0039] After the reaction, 60 ...
Embodiment 3
[0044] The preparation of embodiment 3 diabetes mouse model
[0045] Streptozotocin (STZ) can damage islet cells, resulting in insufficient insulin secretion and hyperglycemia, leading to type Ⅰ diabetes. We used the method of intraperitoneal injection of STZ (50mg / kg) to establish a type Ⅰ diabetes model, selected 8-week-old male C57 mice, injected STZ intraperitoneally for five consecutive days, and tested blood glucose one week later. The mice with blood glucose greater than 11.1mmol / L were returned to For the diabetes group (Wld(S)protectsagainstperipheralneuropathyandretinopathyanexperimentalmodelofdiabetesinmice. Diabetologia. 2011,54(9):2440-50.)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com