Dendritic cell vaccine and preparation method and application of dendritic cell vaccine
A technology of dendritic cells and vaccines, applied in the field of medical bioengineering, can solve problems such as limited expression, and achieve the effect of less toxic side effects and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The integration of embodiment 1 Oct4 and Sox2 gene at AAVS1 site
[0032] Tumor cells exist in heterogeneous populations. Therefore, in order to obtain a consistent effect of genetic engineering of liver cancer cells, the present invention obtains HepG2 of a homologous population for the first time through single cell cloning. Single cells were seeded in 96-well plates, and the holes were enlarged. When its parental cell line HepG2, SC was selected for further application, the clone showed similar morphology and growth rate.
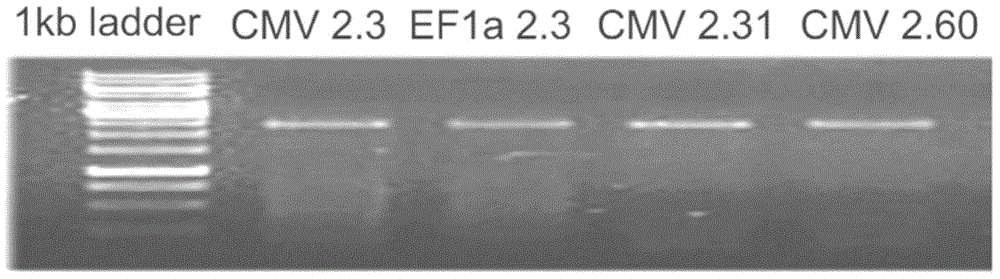
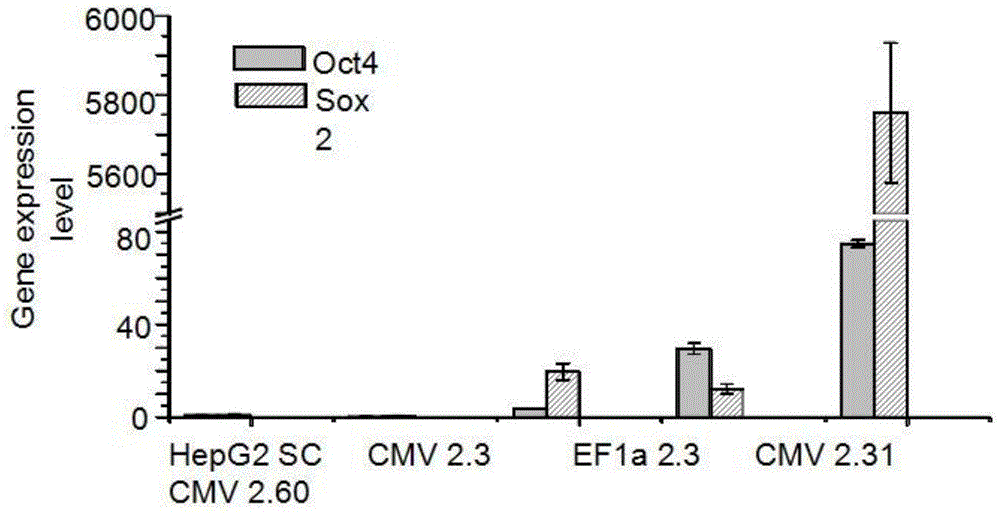
[0033] In order to produce HepG2CSC-like cells, the present invention utilizes baculovirus-mediated zinc finger nuclease technology to introduce gene cassettes containing Oct4 and Sox2 genes (the specific gene sequences of Oct4 and Sox are described in the attached gene sequence table) into the HepG2SC cells AAVS1 locus. Two different promoters, CMV and EF1A, were tested for the expression of these two genes. Liver cancer cells HepG2 and BV-ZF...
Embodiment 2
[0035] Example 2: Preparation of liver cancer cells with expression of cancer stem cell markers
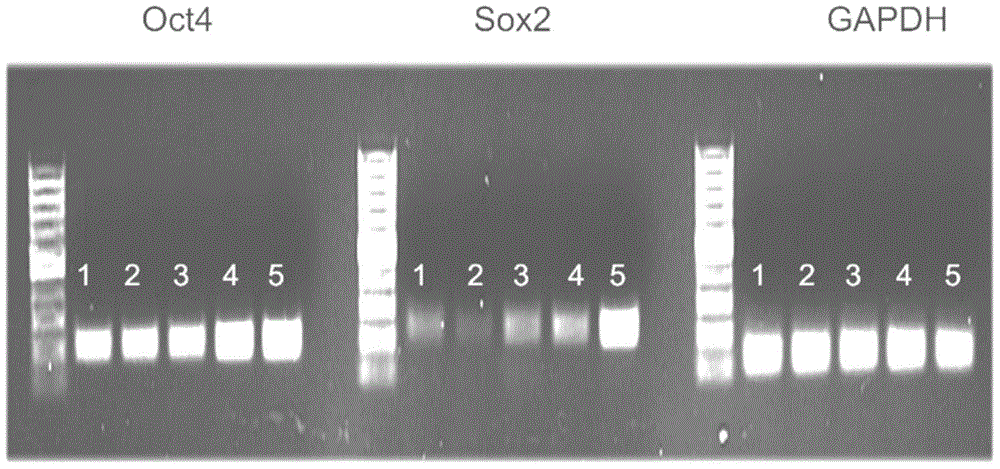
[0036] Routine cell culture was carried out in a humidified incubator with 10% fetal bovine serum (Invitrogen) and 1% penicillin / streptomycin (Invitrogen) in DMEM medium (InvitrogenLife Technologies, Carlsbad, CA) at 37°C and 5% carbon dioxide. . The sphere culture technique is commonly used to aggregate, maintain and expand the number of CSCs from cultured adherent cells. The above-mentioned transgenic liver cancer cells were cultured in spheres to detect whether up-regulating the gene expression of Oct4 and Sox2 can increase the number of CSCs, and the criteria for defining CSCs are the expression profiles and expression levels of marker genes.
[0037] First, human hepatoma HepG2 cells were isolated for single cell cloning. Cells are seeded individually in culture medium in a 96-well culture plate. Each clone was then propagated individually. Human hepatoma cell single cell...
Embodiment 3
[0046] Example 3: DC vaccine against tumor stem cell markers
[0047] Dendritic cells are derived from human peripheral blood mononuclear cells (PBMC). PBMCs are FicollGradientFicoll-Paque TM Technology (GE Healthcare Life Sciences) isolated from fresh buffy coat. Frozen / thawed hepatocellular carcinoma stem cell-producing lysates and human peripheral mononuclear cell-derived dendritic cells and lysates used in the present invention. To study the priming capacity of the resulting dendritic cells, T cells were obtained from easysep TM Peripheral blood mononuclear cells were isolated from human naive CD8+ T cell enrichment kit (Stem Cell Isolation Technology). The immature T cells were cultured in Cellgro medium specially for culturing T cells, adding 5% human AB serum (US Life Technologies), IL-2 (50IU / ml), 20ng / ml IL-7 (for) The ratio of primed naive T cells to mature dendritic cells was 1:2.5, 1:5 or 1:10. Grow and expand on the cell culture medium described above. One w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com