Method for preparing salbutamol compound inhalation aerosol
A technology of salbutamol and levosalbutamol, which is applied in the field of biomedicine, can solve the problems of low bioavailability and poor compliance with clinical medication, and achieve good lung deposition, improve drug compliance, and rapid onset of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1. Formula: calculated according to 1000 bottles:
[0059]
[0060] 2. Preparation method:
[0061] 1) Micronization of raw materials: pulverizing levosalbutamol sulfate and ketotifen fumarate;
[0062] 2) Weighing: take by weighing formula amounts of L-salbutamol sulfate, ketotifen fumarate, oleic acid, absolute ethanol, anhydrous sodium sulfate;
[0063] 3) Surfactant dissolution: Add the surfactant to absolute ethanol, slowly add the formulated amount of levosalbutamol sulfate and ketotifen fumarate, stir and continue until a clear and transparent solution is formed;
[0064] 4) Drying: Add the desiccant in the prescribed amount, stir and dry for 30 minutes, and then filter through a titanium rod to remove the desiccant;
[0065] 5) Filling and tying valves, filling propellant tetrafluoroethane, leak detection and weighing;
[0066] 6) Packaging: install the actuator (cover).
Embodiment 2
[0068] 1. Formula: calculated according to 1000 bottles:
[0069]
[0070]
[0071] 2. Preparation method:
[0072] The preparation method is the same as in Example 1.
experiment example 1
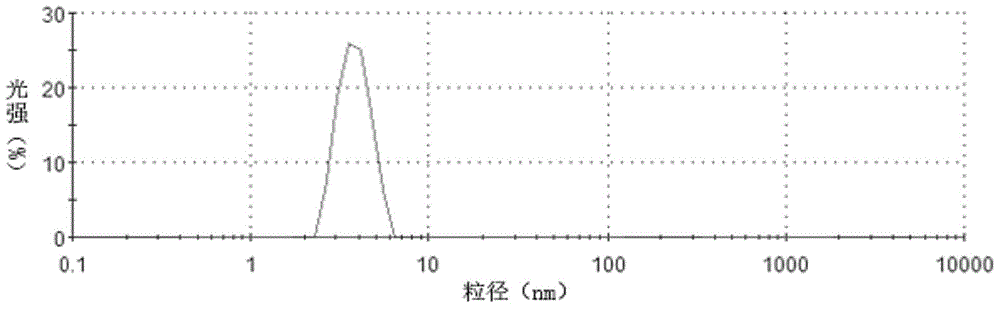
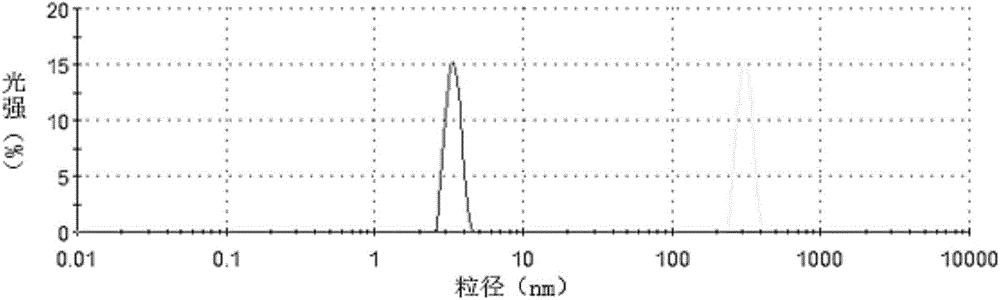
[0073] Experimental Example 1 Particle Size Measurement
[0074] The droplet size distribution was determined by laser diffraction using a Mastersizer 3000 (Malvern Instruments, Worcs, UK). The powder was measured to be dispersed by a Scirocco 3000 aerosol feeder (Malvern Instruments, Worcs, UK) with compressed air at 4 bar. The particles have a refractive index and absorptivity of 1.52 and 0.1, respectively, and the dispersant has a refractive index of 1.000 for air. All measurements were performed in three cycles and the results are shown in Tables 1 and 2 and figure 1 with figure 2 .
[0075] Table 1 Example 1 sample particle size distribution
[0076]
Dv10
Dv50
Dv90
Average particle size (μm)
1.41
3.54
4.87
standard deviation(%)
0.03
0.03
0.05
[0077] Table 2 Example 2 sample particle size distribution
[0078]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com