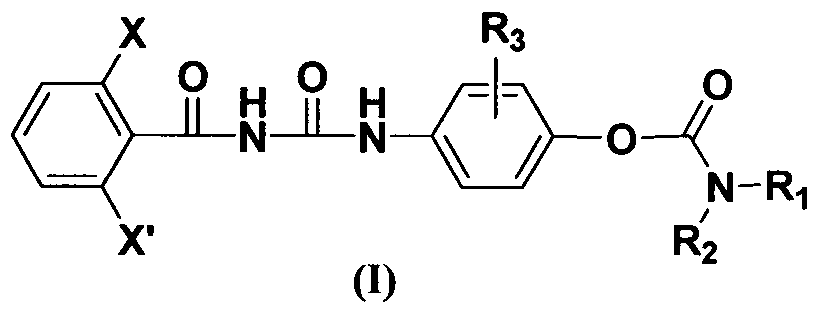
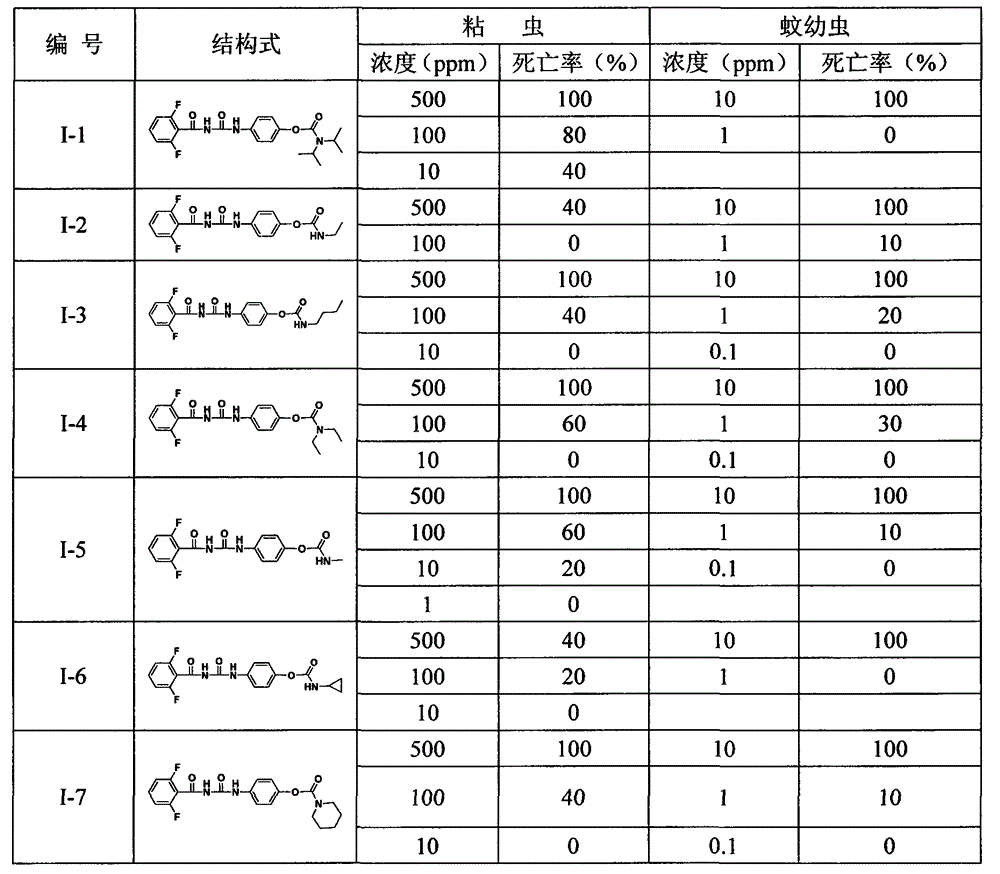
Benzoyl urea compound containing urethane group structure as well as preparation method and insecticidal application of benzoyl urea compound
A carbamate-based, benzoylurea technology, applied in the field of benzoylurea compounds, to achieve the effects of completely novel structure, wide insecticidal activity, and excellent insecticidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
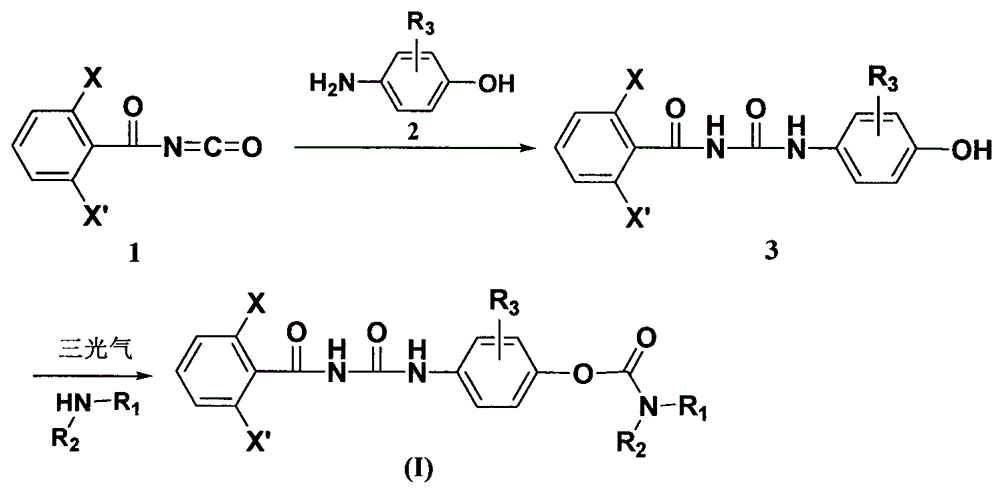
[0016] Embodiment 12, the preparation of 6-difluorobenzoyl-4-hydroxyphenylurea
[0017] Slowly add the acetonitrile solution of 3.0g 2,6-difluorobenzoyl isocyanate dropwise to the acetonitrile solution of 1.8g p-aminophenol and 0.2g triethylamine, stir at room temperature overnight, and use dichloromethane:methanol=10:1 The column obtained 4.5 g of 2,6-difluorobenzoyl-4-hydroxyphenylurea as a gray solid, yield 93.8%, melting point: 160.0-162.0°C. 1 HNMR (400MHz, DMSO): δ11.36(brs, 1H), 9.99(brs, 1H), 7.61(m, 1H), 7.33(d, J=8.5Hz, 2H), 7.25(t, J=8.2Hz , 2H), 6.73(d, J=8.8Hz, 2H), 6.02(s, 1H).
Embodiment 22
[0018] Embodiment 22, Preparation of 6-difluorobenzoyl-4-(N,N-diisopropylcarbamate phenyl)urea
[0019] After stirring 0.5g of 2,6-difluorobenzoyl-4-hydroxyphenyl urea, 1.0g of potassium carbonate in acetonitrile and aqueous solution at room temperature for 0.5 hours, slowly drop in 0.4g of triphosgene in acetonitrile solution, and react again for 0.5 hours and slowly A solution of 0.4 g of diisopropylamine in acetonitrile was added dropwise, and the reaction was stopped after 0.5 hours. After the reaction solution was washed twice with water, the organic phase was dried with anhydrous sodium sulfate, solvent removal, petroleum ether: ethyl acetate = 5: 1 silica gel column chromatography, the above product was obtained as a white solid 0.4 g, yield 55.6%, melting point: 208-209 ℃. 1 HNMR (400MHz, CDCl 3 ): δ10.41(s, 1H), 7.89(s, 1H), 7.50-7.56(m, 3H), 7.05-7.14(m, 4H), 3.98-4.12(m, 2H), 1.33-1.35(m , 12H).
Embodiment 32
[0020] Embodiment 32, the preparation of 6-difluorobenzoyl-4-(piperidine formic acid phenyl) urea
[0021] After stirring 0.5g of 2,6-difluorobenzoyl-4-hydroxyphenyl urea, 1.0g of potassium carbonate in acetonitrile and aqueous solution at room temperature for 0.5 hours, slowly add dropwise 0.4g of triphosgene in acetonitrile solution, and react again for 0.5 hours and slowly A solution of 0.5 g of piperidine in acetonitrile was added dropwise, and the reaction was stopped after 0.5 hours. After the reaction solution was washed twice with water, the organic phase was dried with anhydrous sodium sulfate, precipitated, petroleum ether: ethyl acetate = 2:1 silica gel column chromatography, and the above product was obtained as a white powdery solid 0.1 g, yield 14.5%, melting point: 212 -215°C. 1 HNMR (400MHz, DMSO): δ11.44(s, 1H), 10.19(s, 1H), 7.63(m, 1H), 7.56(d, J=7.6Hz, 2H), 7.26(m, 2H), 7.10 (d, J = 7.9 Hz, 2H), 3.50-3.60 (m, 2H), 3.45-3.55 (m, 2H), 1.55-1.59 (m, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com