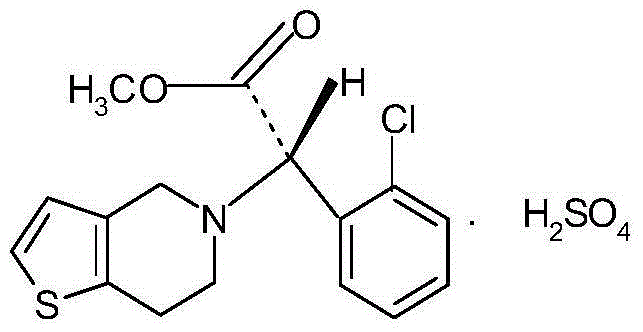
Clopidogrel hydrogen sulphate tablets and preparation method thereof
A technology of clopidogrel bisulfate tablets and clopidogrel bisulfate, which is applied in the fields of pharmaceutical formula, drug delivery, blood diseases, etc., and can solve the problems of poor solubility, difficulty in disintegration and dissolution of clopidogrel bisulfate, etc. Achieve the effects of high dissolution, high production efficiency and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
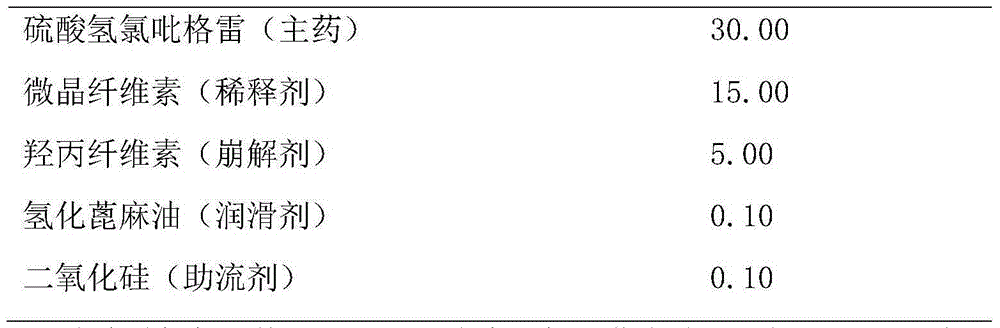
[0019] The preparation specification of embodiment 1 clopidogrel bisulfate tablet is 75 mg (calculated by clopidogrel)
[0020] Table 1 is calculated per 1000 pieces
[0021]
[0022]
[0023] Preparation process: Pass clopidogrel hydrogen sulfate, hydroxypropyl cellulose, hydrogenated castor oil, and silicon dioxide through a 80-mesh sieve and disperse microcrystalline cellulose through a 60-mesh sieve, respectively weigh the above-mentioned raw and auxiliary materials according to the prescription amount, and set aside. First mix clopidogrel bisulfate, hydroxypropyl cellulose, silicon dioxide and microcrystalline cellulose in advance, then add hydrogenated castor oil and mix evenly, the powder is directly compressed into tablets to obtain plain tablets, and Opadry is formulated into 16% Aqueous solution, the weight of the coating is increased to 1%, and the product is obtained.
[0024] Measure the clopidogrel bisulfate agent prepared by the embodiment of the inventio...
Embodiment 2
[0027] The preparation specification of embodiment 2 clopidogrel bisulfate tablet is 75mg (according to clopidogrel)
[0028] Table 3 is calculated per 1000 pieces
[0029]
[0030] Preparation process: Pass clopidogrel hydrogen sulfate, hydroxypropyl cellulose, hydrogenated castor oil, and silicon dioxide through a 80-mesh sieve and disperse microcrystalline cellulose through a 60-mesh sieve, respectively weigh the above-mentioned raw and auxiliary materials according to the prescription amount, and set aside. First mix clopidogrel bisulfate, hydroxypropyl cellulose, silicon dioxide and microcrystalline cellulose in advance, then add hydrogenated castor oil and mix evenly, the powder is directly compressed into tablets to obtain plain tablets, and Opadry is formulated into 16% Aqueous solution, the weight of the coating is increased to 1%, and the product is obtained.
[0031] Measure the clopidogrel bisulfate prepared by the embodiment of the invention 2 according to the...
Embodiment 3
[0034] Example 3 Preparation of Clopidogrel Hydrogen Sulfate Tablet Specifications 75mg (calculated as clopidogrel)
[0035] Table 5 is calculated per 1000 pieces
[0036]
[0037] Preparation process: Pass clopidogrel hydrogen sulfate, hydroxypropyl cellulose, hydrogenated castor oil, and silicon dioxide through a 80-mesh sieve and disperse microcrystalline cellulose through a 60-mesh sieve, respectively weigh the above-mentioned raw and auxiliary materials according to the prescription amount, and set aside. First mix clopidogrel bisulfate, hydroxypropyl cellulose, silicon dioxide and microcrystalline cellulose in advance, then add hydrogenated castor oil and mix evenly, the powder is directly compressed into tablets to obtain plain tablets, and Opadry is formulated into 16% Aqueous solution, the weight of the coating is increased to 1%, and the product is obtained.
[0038] Measure the clopidogrel bisulfate prepared by the embodiment of the invention 3 according to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com