A method for simultaneously removing heavy metals-organic compound polluted water bodies using zero-valent iron-persulfate
A technology of persulfate and compound pollution, applied in water pollutants, chemical instruments and methods, reduced water/sewage treatment, etc. Simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of Bentonite-loaded Nano Zero-Valent Iron
[0030] In a 200mL three-neck flask, add 50mL of the mixed solution (V 乙醇 :V 水=4:1), weighed 9.66g of ferric chloride hexahydrate and added to the mixed solution and stirred until completely dissolved. Next, weigh 2 g of bentonite and add it to a three-necked flask, and stir for 10 minutes under nitrogen protection to ensure complete and uniform mixing. Subsequently, prepare 100 mL of 0.93M NaBH 4 Solution, under the protection of nitrogen, add NaBH dropwise 4 The solution was added to the mixed solution in the three-necked flask, and continued to stir for 20min to make the NaBH 4 Reacts completely with ferric chloride to ensure Fe 3+ Completely reduced to zero valent iron. After the reaction was completed, the black solid in the three-neck flask was recovered by vacuum filtration, washed with ethanol, placed in a vacuum oven, dried at 75°C for 600 min, and then stored in a brown bottle filled with ...
Embodiment 2
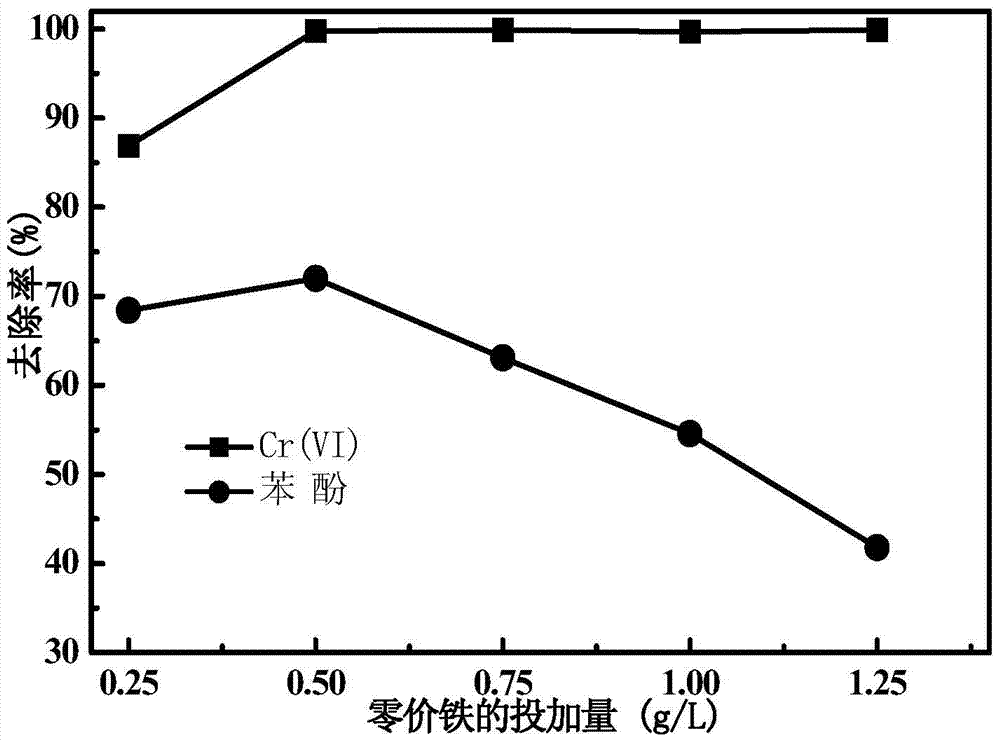
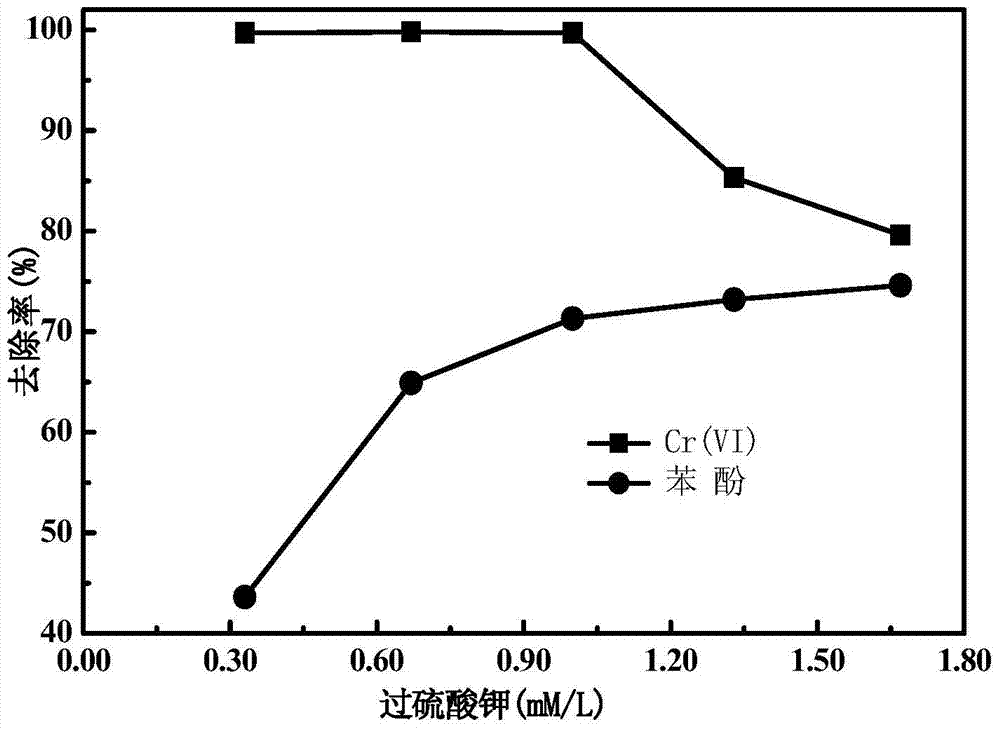
[0032] The joint reaction of bentonite-loaded nano-zero-valent iron and potassium persulfate is adopted, that is, bentonite-loaded nano-zero-valent iron and potassium persulfate are simultaneously added to water containing Cr(Ⅵ) and phenol to remove water pollutants. The specific steps are: use a 100mL triangular flask as a reactor, and treat 30mL of water with a concentration of 20mg / L Cr(Ⅵ) and 10mg / L phenol, and the pH of the water is not adjusted, which is displayed as 5.6. Add potassium persulfate to reactor, make its final concentration be 1.00mM and the bentonite loaded nanometer zero-valent iron prepared by embodiment 1, make its final concentration be 0.50g / L, and reactor is placed on the oscillating stirrer, The rotating speed is 160rpm, and the reaction time is 30min. The specific results are shown in Table 1.
Embodiment 3
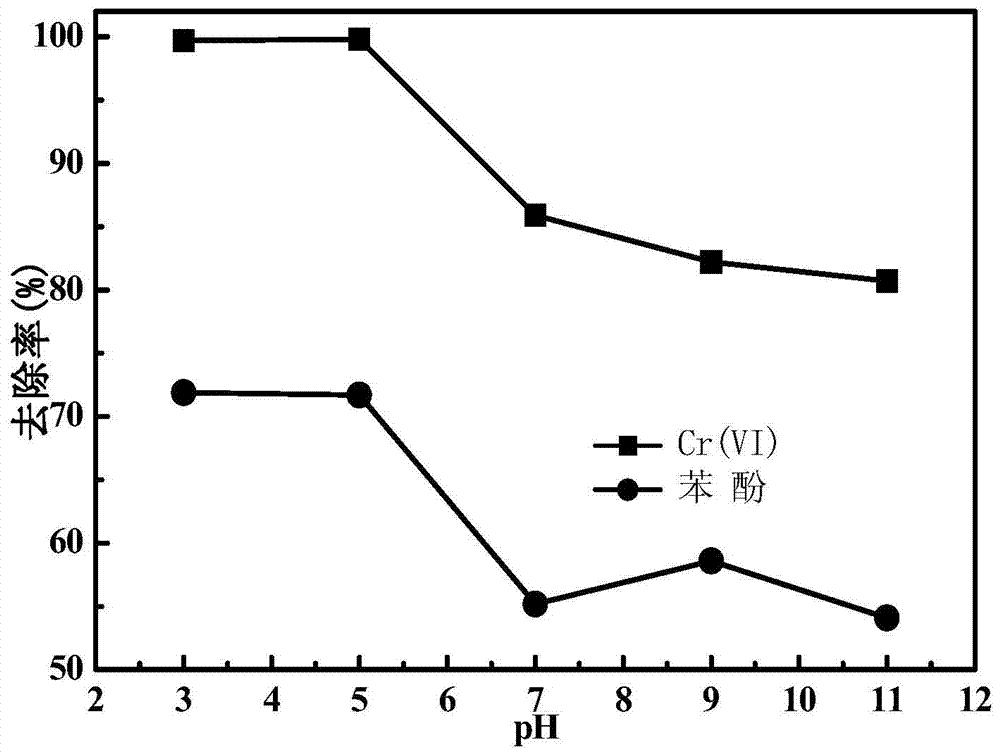
[0044] A 100mL triangular flask was used as the reactor, and the treatment object was 30mL of phenol water with a concentration of 10mg / L, and the pH of the water was adjusted to 3, 5, 7, 9, and 11, respectively. Add potassium persulfate to the reactor containing the water body of various pH values respectively, make its final concentration be 1.00mM and the bentonite loaded nanometer zero-valent iron prepared by embodiment 1, make its final concentration be 0.50g / L, and The reactor was placed on an oscillating stirrer with a rotating speed of 160 rpm and a reaction time of 30 min, and the changes in the concentrations of Cr(VI) and phenol in the water before and after the reaction were measured. The result is as figure 1 shown, from figure 1 It can be seen that in the bentonite-supported nano-zero-valent iron-potassium persulfate joint system, after 30 minutes of reaction, the removal rate of Cr(Ⅵ) and phenol reached the maximum in the pH range of 3-5, and the removal rate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com