Acinetobacter baumannii subunit vaccine antigen protein, and applications thereof
A technology of Acinetobacter baumannii and subunit vaccine, which is applied in the fields of molecular biology and immunology, and can solve the problems such as mixing of irrelevant proteins and impurities of active ingredients, complex Baumannian drug resistance mechanism, and difficulty in improving yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Construction of the prokaryotic expression plasmid for the outer membrane protein OmpW of Acinetobacter baumannii
[0032] The Acinetobacter baumannii strain ATCC17978 was cultured overnight in LB medium until the bacteria grew to the logarithmic phase (OD 600 =0.4-0.6), the bacterial solution was taken for PCR amplification, and the PCR amplification primer was OmpW-F (5' GGATCC ATGGGGGTGTCTTCATTTACTT3') and OmpW-R (5' GAATTCTTAGAATTTATAGCTATAGCCT3'); the specific PCR system is: bacteria solution: 2 μL; OmpW-F: 2 μL; OmpW-R: 2 μL; dNTP: 2 μL; Taq enzyme: 0.25 μL; 10×Taq enzyme buffer: 2.5 μL; water: 14.25 μL. The PCR program was: ①pre-denaturation at 94°C for 5 minutes; ②denaturation at 94°C for 30 seconds; annealing at 56°C for 30 seconds; extension at 72°C for 1 minute, 35 cycles; ③final extension at 72°C for 10 minutes. The PCR product was gel-recovered (Beijing Tiangen Biology), and the recovered product was connected to the pMD19T-simple vector (Tak...
Embodiment 2
[0033] Embodiment 2: Induced expression of Acinetobacter baumannii OmpW recombinant protein
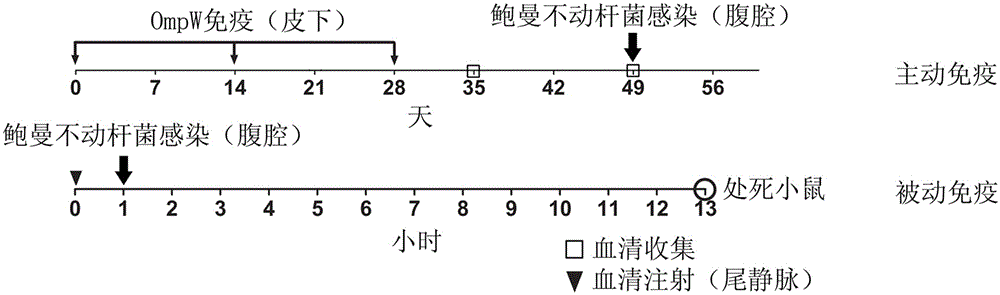
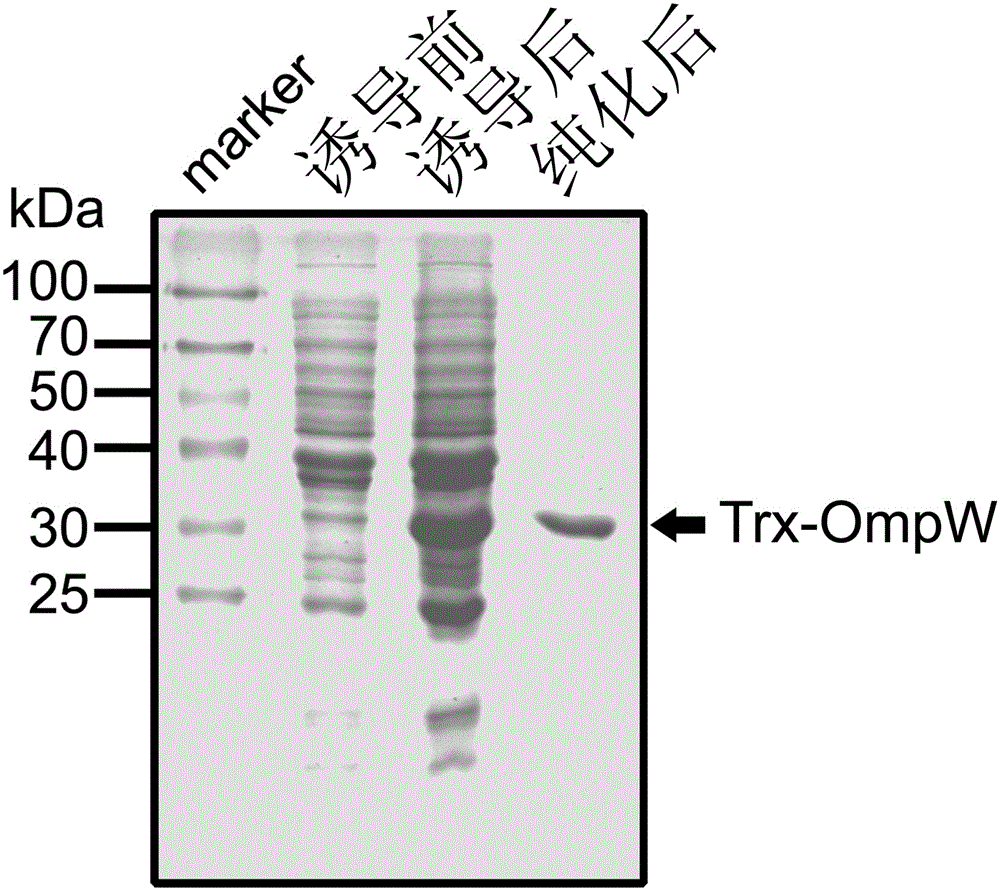
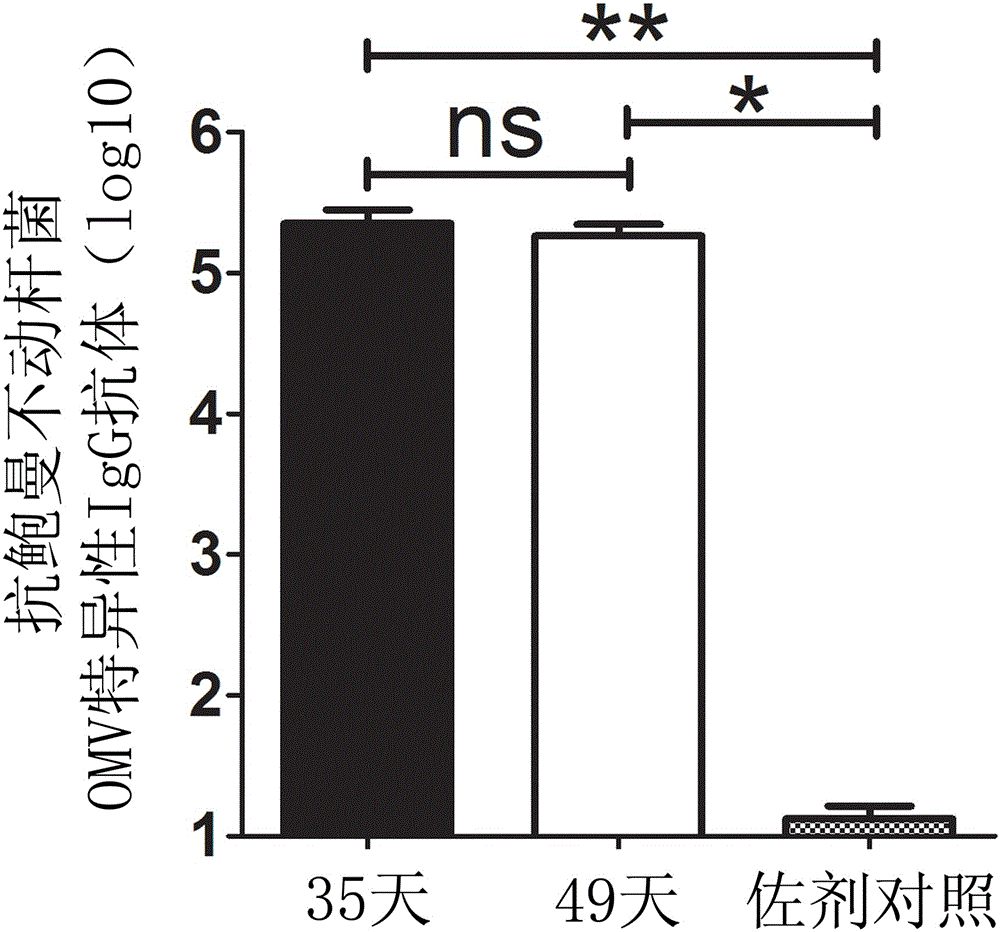
[0034] Transform the recombinant plasmid into BL21(DE3) competent cells, and pick a single clone to induce expression. The specific method is to inoculate the positive clone into LB medium that has been added with ampicillin (100 mg / mL), and culture it for 16 hours. Transfer the bacterial liquid into the new LB medium with ampicillin at a ratio of 1:5. When the strain grows to the logarithmic phase, add IPTG (1mmol / L) to induce expression, and control the induction expression temperature to 30-37°C , induced expression for 6 hours ( figure 2 ).
Embodiment 3
[0035] Example 3: Preparation and purification of OmpW subunit vaccine antigen
[0036] Centrifuge the sample after overnight induction at 12,000 g at room temperature for 10 minutes to collect the bacteria, wash the bacteria twice with PBS, and then ultrasonically disrupt the cells at 10% maximum power, work for 5 seconds, stop for 5 seconds, and last for 10 minutes. The crushed sample was centrifuged at 12000g for 10 minutes, and the inclusion body precipitate was collected. The inclusion body was washed 3 times with Washbuffer (20mMtris-Hcl, pH8.8, 1M urea) and then added to Solutionbuffer (20mMtris-Hcl, pH8.8, 8M urea). Dissolved, the dissolved inclusion bodies were dialyzed with Bindingbuffer (20mMtris-Hcl, pH8.8) at 4°C overnight for refolding, and the dialysate was purified with an anion-exchange chromatography column HiTrapQFF (GE Healthcare), and filtered through Elutionbuffer (20mMtris-Hcl, pH8 .8, 2M Nacl) after gradient elution, the purified products of different c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com