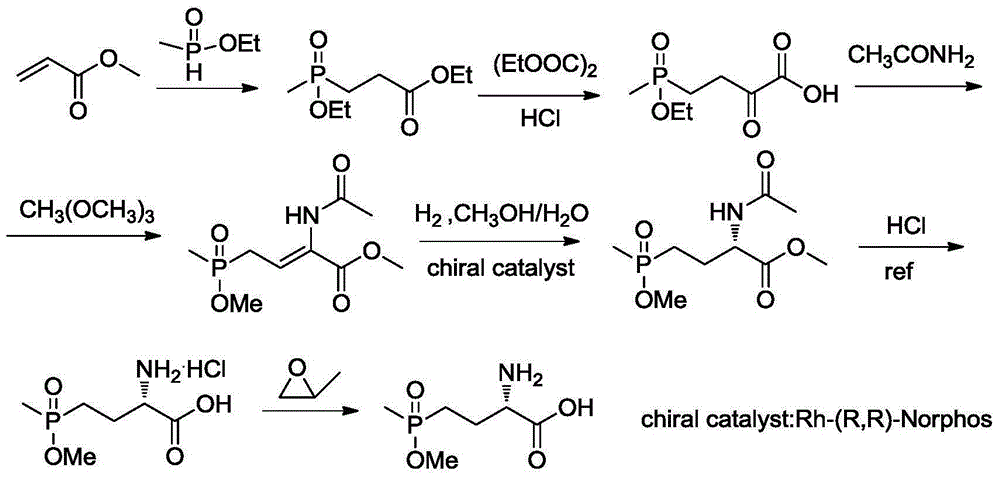
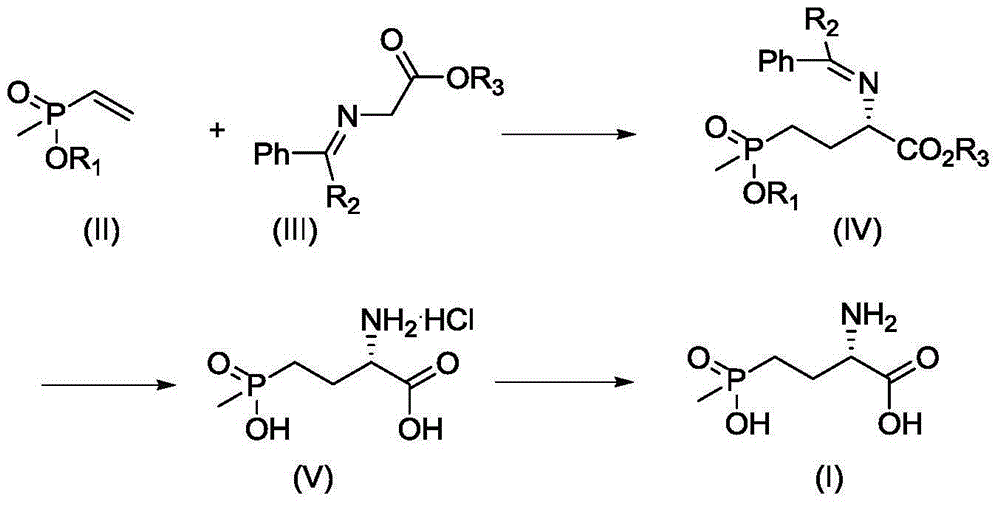
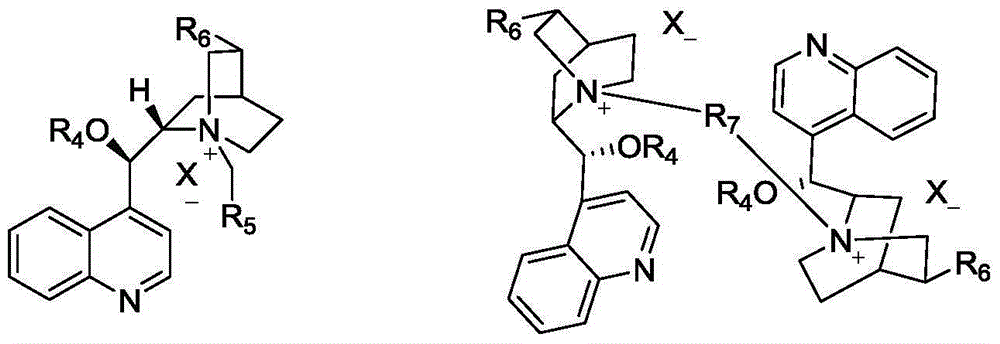
Synthetic method for L-phosphinothricin
A glufosinate-ammonium synthesis reaction technology, applied in the field of pesticide chemical herbicide synthesis, can solve the problems of high price, difficult substrate synthesis, unsuitable for industrial production, etc., and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1)
[0034]
[0035] In a 50mL three-neck flask, add 2.53g (0.01mol) of dibenzylidene glycine methyl ester, 3.26g (0.01mol) of cesium carbonate, 0.52g (0.001mol) of catalyst and 15mL of dichloromethane in sequence, and cool the system down to -20°C , slowly drop into a solution of 1.2g (0.01mol) of methyl vinyl phosphonate in dichloromethane (5mL), react at -20°C, monitor the reaction in the liquid phase until the reaction of dibenzylidene glycine methyl ester is complete, filter, Filtrate desolvation column chromatography (catalyst recovery) to obtain colorless oily liquid (S)-2-diphenylmethylene amino-4-methoxy (methyl) phosphonobutanoic acid methyl ester 2.51g, yield 66.7%, 1 HNMR (500MHz, CDCl 3 ):7.60-7.63(dd,2H,2×Ar-H),7.43-7.45(m,3H,3×Ar-H),7.36-7.39(t,1H,Ar-H),7.30-7.33(t ,2H,2×Ar-H),7.14-7.17(dd,2H,2×Ar-H),3.95-3.96(m,1H,CH),3.63-3.66(dd,3H,OCH 3 ),3.85(s,3H,OCH 3 ),2.04-2.15(m,2H,CH 2 ),1.75-1.86(m,2H,CH 2 ), 1.42-1.46 (d, 3H, J=3.8Hz, CH 3 ). 31 ...
Embodiment 2
[0040] (1)
[0041]
[0042] In a 50mL three-neck flask, add 2.95g (0.01mol) tert-butyl dibenzylidene glycine, 0.56g (0.01mol) potassium hydroxide, 0.488g (0.001mol) catalyst and 15mL toluene in sequence, and cool the system down to -10°C , slowly drop 1.2g (0.01mol) of methyl vinyl phosphonate in toluene (5mL) solution, react at -10°C, monitor the reaction in the liquid phase until the reaction of tert-butyl dibenzylidene glycine is completed, filter, and the filtrate Desolvation column chromatography (catalyst recovery) gave 2.66 g of colorless oily liquid (S)-2-diphenylmethyleneamino-4-methoxy (methyl) phosphonobutanoic acid tert-butyl ester, yield 64.1%, 1 HNMR (500MHz, CDCl 3 ):7.63-7.64(dd,2H,2×Ar-H),7.44-7.45(m,3H,3×Ar-H),7.38-7.41(t,1H,Ar-H),7.31-7.34(t ,2H,2×Ar-H),7.16-7.18(dd,2H,2×Ar-H),3.94-3.96(m,1H,CH),3.67-3.69(dd,3H,OCH 3 ),2.06-2.16(m,2H,CH 2 ),1.74-1.86(m,2H,CH 2 ),1.44-1.46(m,12H,CH 3 ,t-Bu). 31 PNMR (500MHz,D 2 O): 56.6.
[0043] (2)
[0044] ...
Embodiment 3
[0047] (1)
[0048]
[0049] In a 50mL three-necked flask, add 2.95g (0.01mol) tert-butyl dibenzylidene glycine, 0.96g (0.01mol) sodium tert-butoxide, 0.511g (0.001mol) catalyst and 15mL tetrahydrofuran, and cool the system to -10 ℃, slowly drop 1.2g (0.01mol) tetrahydrofuran (5mL) solution of methyl vinyl phosphonate, react at -10 ℃, monitor the reaction in the liquid phase until the reaction of tert-butyl dibenzylidene glycine is completed, filter, The filtrate was desolvated by column chromatography (catalyst recovery) to obtain 2.59 g of colorless oily liquid (S)-2-diphenylmethyleneamino-4-methoxy(methyl)phosphonobutanoic acid tert-butyl ester. The rate is 62.4%.
[0050] (2)
[0051]
[0052] Add 2g (0.0048mol) (S)-2-diphenylmethyleneamino-4-methoxy(methyl)phosphonobutanoic acid tert-butyl ester into 40mL hydrochloric acid solution (6mol / L), the reaction solution Raise the temperature to reflux, monitor the liquid phase until the reaction of the raw materials is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com