Cross-linked polystyrene microsphere immobilized tetramethylpiperidine nitroxide free radical catalyst and its preparation and application method
A technology of tetramethylpiperidine nitroxide radical and cross-linked polystyrene, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc. development and other issues to achieve the effect of improving thermal stability and facilitating separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
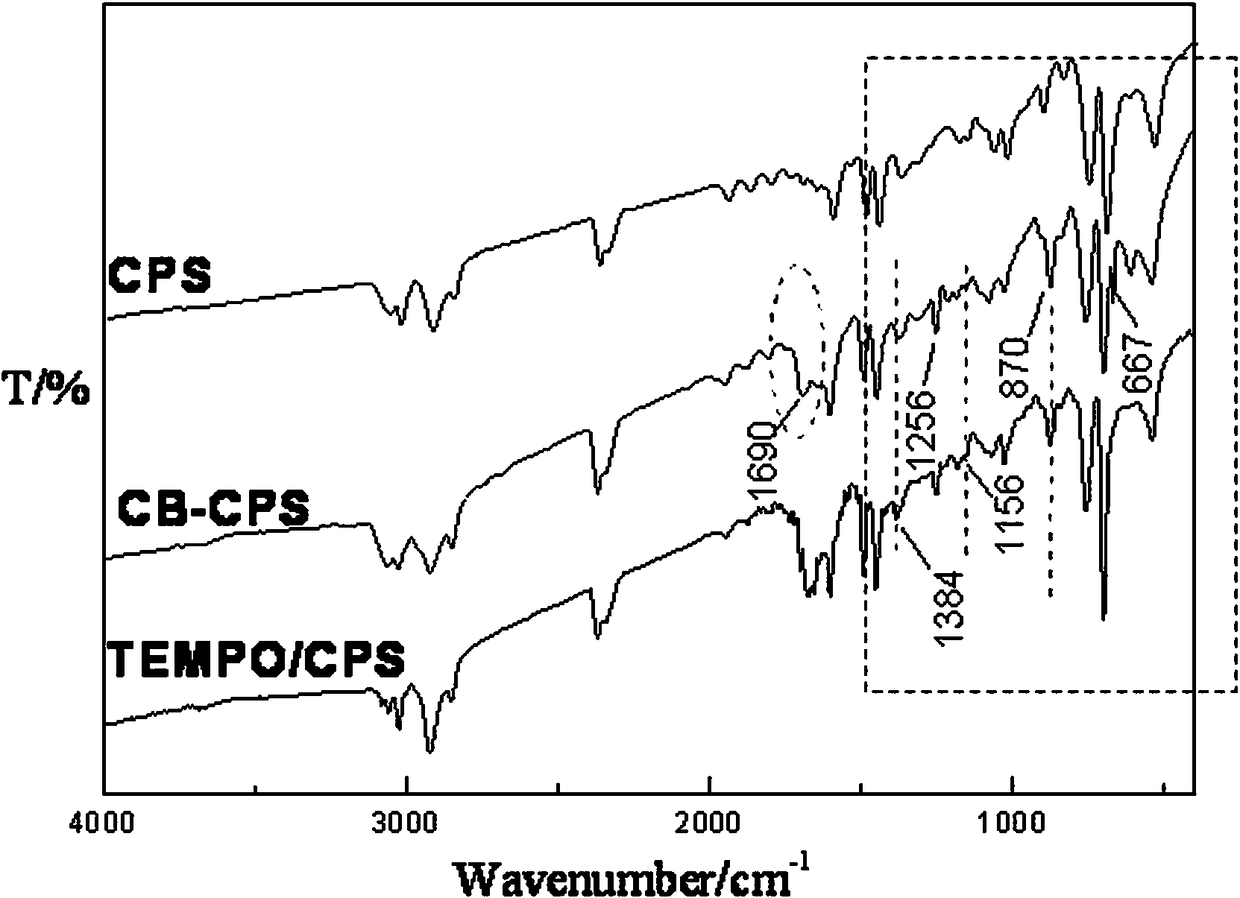
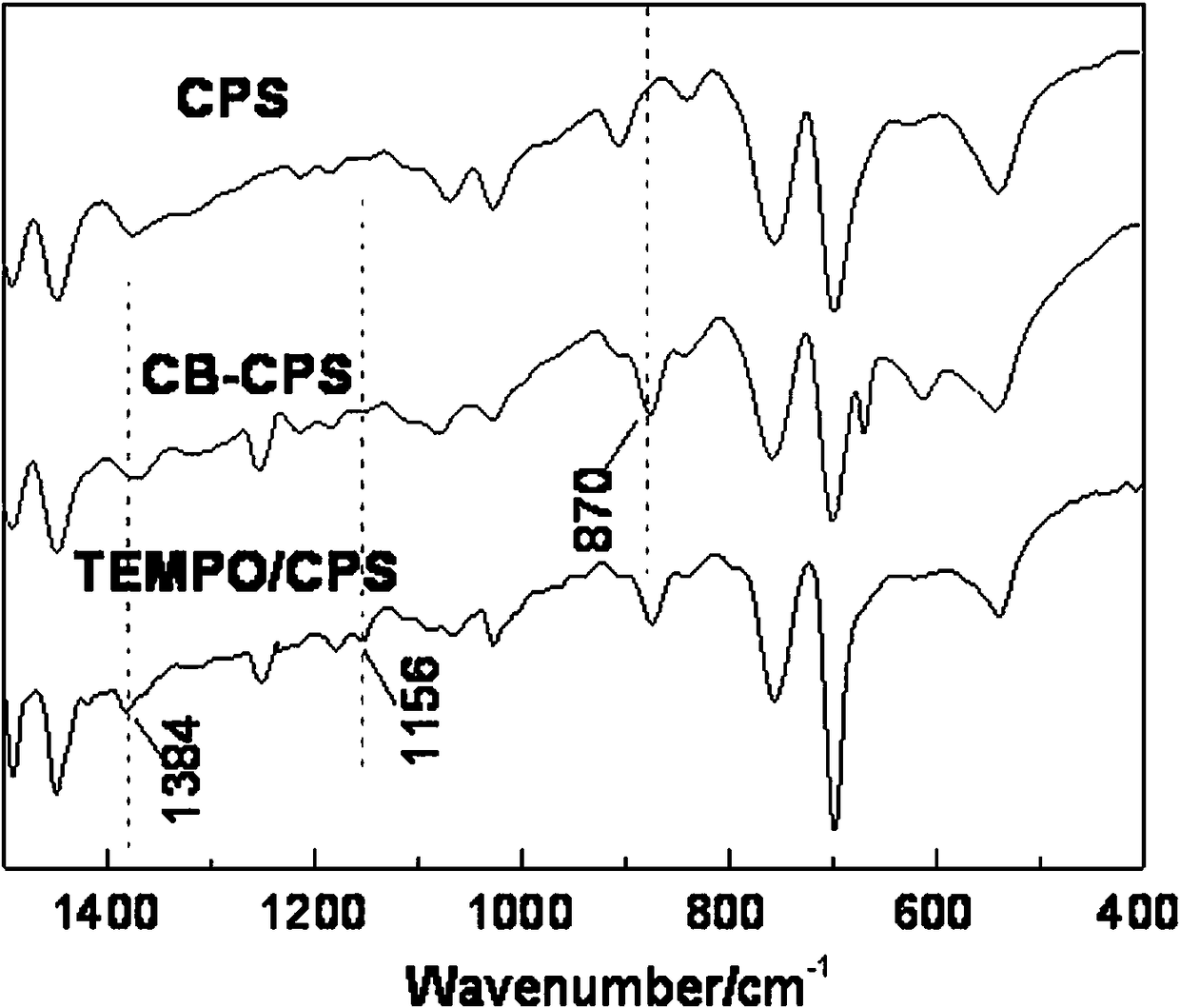
[0021] Example 1: In a four-neck flask, add 2.0 g of CPS microspheres and place them in 20 mL of chloroform, soak for 12 hours to fully swell the microspheres, add 2.6 g of AlCl 3 ; Use a constant pressure dropping funnel to slowly add 13 mL of chloroform solution dissolved with 2.2 mL of chlorobutyryl chloride dropwise, heat up to 75°C, react at a constant temperature for 5 hours, filter, wash the product microspheres with dilute hydrochloric acid, 1,4-dioxane and ethanol The microspheres were washed repeatedly, and then washed with distilled water until there was no chloride ion in the washing liquid, and dried in vacuum to constant weight to obtain the chlorobutyrylated microspheres CB-CPS. The bonding amount of chlorobutyryl on the surface of microsphere CB-CPS is 2.9 mmol / g.
[0022] In a four-neck flask, add 1.0 g of microspheres CB-CPS and 21 mL of DMF, soak for 12 h to fully swell the microspheres; weigh 2.1 g of 4-OH-TEMPO, dissolve it with 30 mL of DMF, add it to the...
Embodiment 2
[0023] Example 2: In a four-necked flask, add 1.9 g of CPS microspheres and place them in 22 mL of carbon tetrachloride, soak for 12 hours to fully swell the microspheres, add 2.6 g of SnCl 4 ; Use a constant pressure dropping funnel to slowly add 10 mL of carbon tetrachloride solution dissolved with 2.2 mL of chlorobutyryl chloride dropwise, raise the temperature to 75°C, react at a constant temperature for 5 hours, filter, wash the product microspheres with dilute hydrochloric acid, and 1,4-dioxane The microspheres were repeatedly washed with ring and ethanol, and then washed with distilled water until there was no chloride ion in the washing liquid, and dried in vacuum to constant weight to obtain the chlorobutyrylated microspheres CB-CPS. The bonding amount of chlorobutyryl groups on the surface of microspheres CB-CPS is 0.9 mmol / g.
[0024] In a four-necked flask, add 1.0 g of microspheres CB-CPS and 23 mL of dimethyl sulfoxide, soak for 12 hours to fully swell the micros...
Embodiment 3
[0025]Example 3: In a four-neck flask, add 2.1 g of CPS microspheres and place them in 25 mL of 1,4-dioxane, soak for 12 hours to fully swell the microspheres, add 2.4 g of AlCl 3 ; Slowly add 11 mL of 1,4-dioxane solution with 2.1 mL of chlorobutyryl chloride dropwise with a constant pressure dropping funnel, heat up to 70°C, react at constant temperature for 4 hours, filter, and wash the product microspheres with dilute sulfuric acid, 1,4 - Wash the microspheres with dioxane and ethanol several times, then wash them with distilled water until there is no chloride ion in the washing solution, and dry them in vacuum to constant weight to obtain the chlorobutyrylated microspheres CB-CPS. The bonding amount of chlorobutyryl on the surface of microsphere CB-CPS is 1.6 mmol / g.
[0026] In a four-neck flask, add 1.0 g of microspheres CB-CPS and 25 mL of N,N-dimethylacetamide, soak for 12 h to fully swell the microspheres; weigh 1.9 g of 4-OH-TEMPO, and use 32 mL of N, After dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com