A kind of sba-15 immobilized 2-azaadamantane nitroxide radical catalyst and its preparation and application
A nitroxide radical and azaadamantane technology is applied in the field of mesoporous molecular sieve SBA-15 immobilized 2-azaadamantane nitroxide radical catalyst, which can solve the complex synthesis process, practical application restriction and high synthesis cost. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
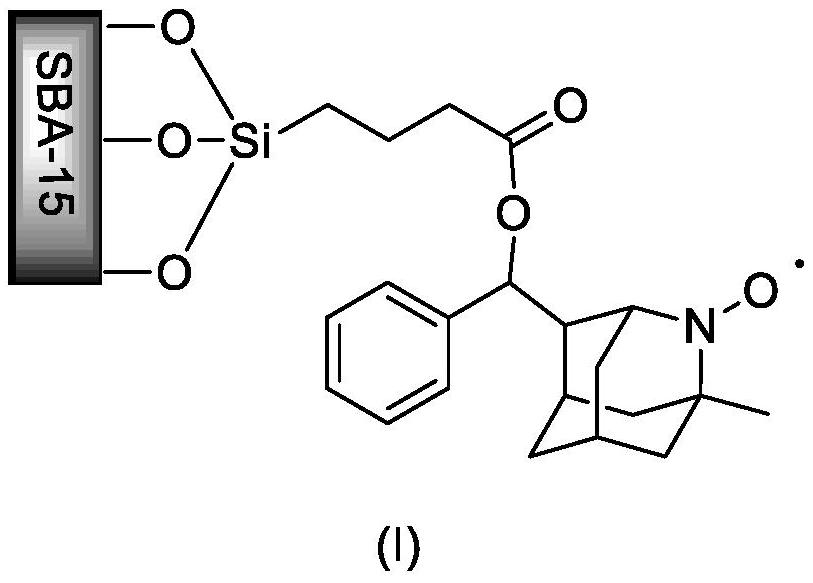
[0021] Example 1: Preparation of SBA-15 Immobilized 2-Azaadamantane-Nitroxy Free Radical Catalyst
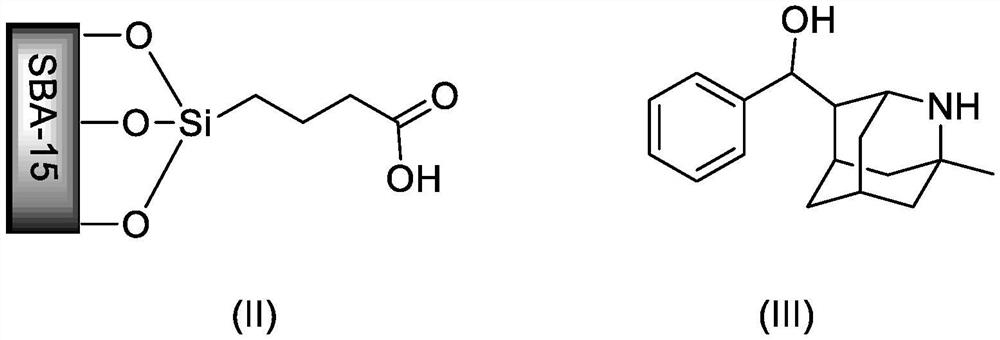
[0022] Add 1.0 g of surface carboxylated SBA-15, 84 mg of 1-methyl-2-azaadamantyl-4-(phenyl)methanol (0.33 mmol) to a 100 mL one-necked bottle equipped with a magnetic stirrer, EDCI (0.66mmol), DMAP (0.36mmol), replace the air in the reaction flask with nitrogen, and inject triethylamine (0.66mmol) and 15mL of dichloromethane. Stir the reaction at room temperature for 24 hours, filter, wash with dichloromethane, and vacuum-dry to constant weight to obtain grafted SBA-15.
[0023] Add the grafted SBA-15 obtained above and 15 mL of tetrahydrofuran into a 50 mL single-necked bottle equipped with a magnetic stirrer, slowly add m-CPBA (0.66 mmol), react at room temperature for 4.5 h, filter, wash with dichloromethane, Vacuum dried to constant weight to obtain SBA-15 immobilized 2-azaadamantane-nitroxyl radical catalyst.
Embodiment 2
[0024] Example 2: Preparation of SBA-15 Immobilized 2-Azaadamantane-Nitroxy Free Radical Catalyst
[0025] The preparation process is the same as in Example 1, except that the amount of 1-methyl-2-azadamantyl-4-(phenyl)methanol is changed to 64mg (0.25mmol), the amount of EDCI is changed to (0.6mmol), and the amount of DMAP is changed to (0.23mmol), the organic base was changed to diisopropylethylamine (0.5mmol), and the amount of m-CPBA was changed to 0.6mmol. Finally, the SBA-15 immobilized 2-azaadamantane-nitrogen radical catalyst was prepared.
Embodiment 3
[0026] Example 3: Application of SBA-15 Immobilized 2-Azaadamantane-Nitroxy Free Radical Catalyst in Alcohol Oxidation
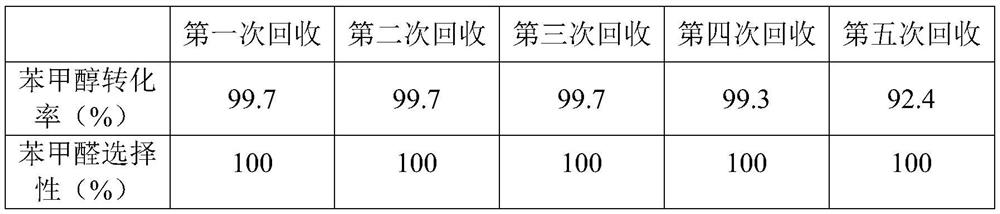
[0027] Add benzyl alcohol (1mmol), KBr (0.1mmol), SBA-15 immobilized 2-azadamantane-nitroxyl radical catalyst 0.1g (prepared in Example 1) to the 15mL sealed tube equipped with a magnetic stirrer , acetonitrile 1 mL, saturated NaHCO 3 Solution 0.7mL. Place the sealed tube in a 5°C ice-water bath, and slowly add NaClO and NaHCO dropwise 3 Add NaHCO in the mixed solution (concentration is 7.5% NaClO solution 1.67g (1.7mmol) 3 Saturated solution 1.2mL). After the dropwise reaction was completed, the reaction was carried out for 10 minutes. Samples were taken and monitored by GC. The conversion rate of benzyl alcohol was 99.7%, and the selectivity of the product benzaldehyde was 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com