Nickel-cobalt-phosphorus crystal, and preparation method and application thereof
A nickel-cobalt-phosphorus crystal technology, applied in the field of nickel-cobalt-phosphorus crystals and its preparation, can solve problems such as poor catalytic activity, achieve stable catalytic performance, good catalytic activity, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention also provides a method for preparing nickel, cobalt and phosphorus crystals, including:
[0042] Mix the nickel source, cobalt source, solvent and phosphorus source to obtain a mixed solution,
[0043] The molar ratio of the nickel source, cobalt source, and phosphorus source is x: (2-x): 2.2, where 0<x<2;
[0044] The obtained mixed solution is heated and reacted to obtain the nickel cobalt phosphorus crystal represented by formula (I),
[0045] Ni x Co (2-x) P formula (I);
[0046] Among them, 0
[0047] According to the present invention, in the present invention, a nickel source, a cobalt source, a phosphorus source and a solvent are mixed to obtain a mixed solution; the nickel source is preferably one or more of nickel acetylacetonate, nickel chloride and nickel nitrate, more preferably Nickel acetylacetonate, nickel chloride or nickel nitrate; the cobalt source is preferably one or more of cobalt acetylacetonate, cobalt chloride and cobalt nitrate...
Embodiment 1
[0055] Weigh 0.064 g nickel acetylacetonate and 0.064 g cobalt acetylacetonate into a 100 mL three-necked flask, dissolve them in 2 mL of octadecene and 3 mL of oleylamine, and add 1 mL of tri-n-octyl phosphine. Stir at room temperature for 15 minutes to mix the reactants uniformly to obtain a mixed solution;
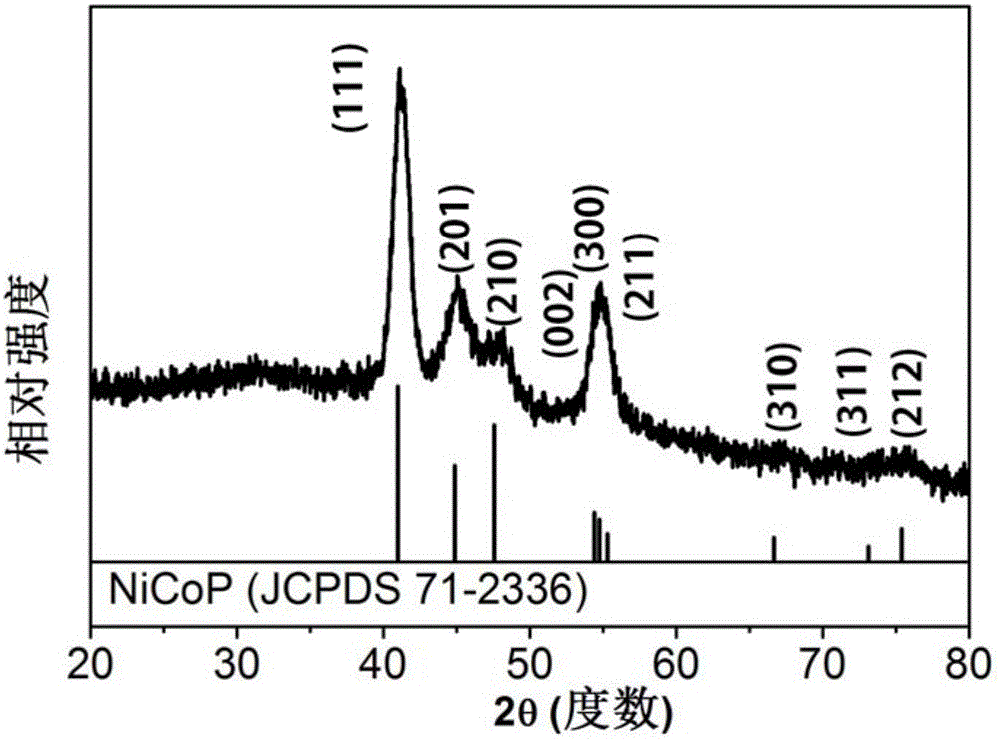
[0056] Under the protection of argon, the mixed solution was heated to 140°C and kept for 30 minutes to remove the low-boiling solvent in the reaction, and then heated to 280°C and maintained at this temperature for 120 minutes. After the reaction, the mixture was naturally cooled to room temperature to obtain a black color. Product, the obtained black product is centrifuged, washed with toluene and absolute ethanol to obtain nickel cobalt phosphorus crystals (NiCoP), and the obtained nickel cobalt phosphorus crystals are dispersed in n-hexane and stored for later use.
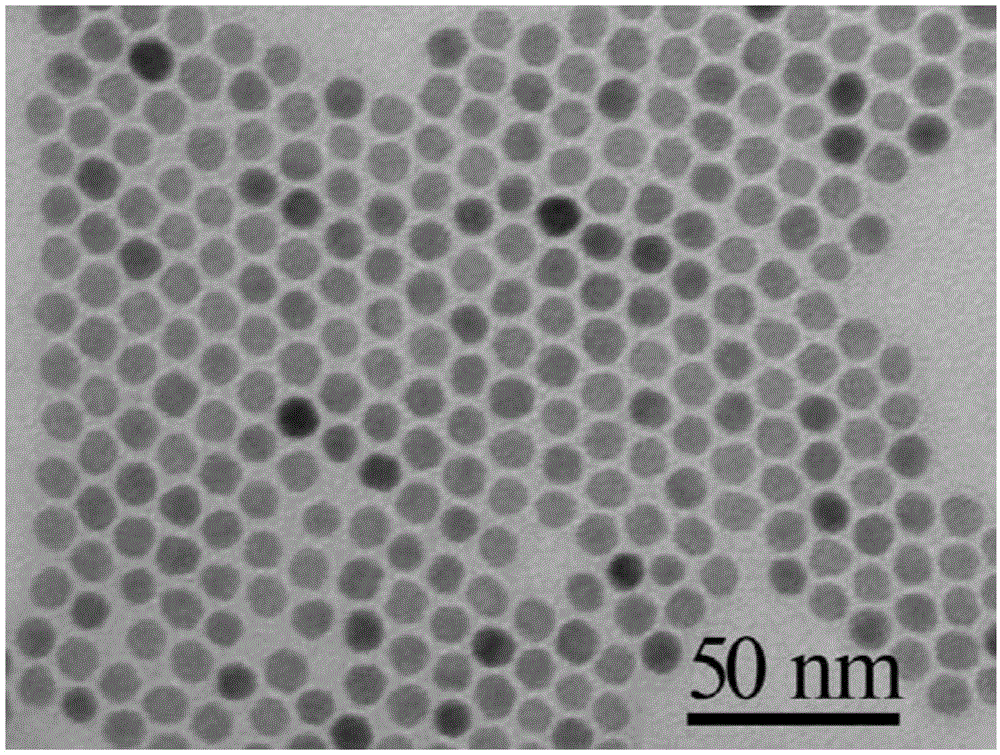
[0057] Observe the obtained nickel, cobalt and phosphorus crystals with a transmission electron microscop...
Embodiment 2
[0068] Weigh 0.032 g nickel acetylacetonate and 0.096 g cobalt acetylacetonate into a 100 mL three-necked flask, dissolve them in 2 mL of octadecene and 3 mL of oleylamine, and add 1 mL of tri-n-octyl phosphine. Stir at room temperature for 15 minutes to mix the reactants uniformly to obtain a mixed solution;
[0069] Under the protection of argon, the mixed solution was heated to 140°C and kept for 30 minutes to remove the low-boiling solvent in the reaction, and then heated to 280°C and maintained at this temperature for 120 minutes. After the reaction, the mixture was naturally cooled to room temperature to obtain a black color. The resulting black product is centrifuged and washed with toluene and absolute ethanol to obtain nickel, cobalt and phosphorus crystals (Ni 0.5 Co 1.5 P), disperse the obtained nickel, cobalt and phosphorus crystals in n-hexane and store them for later use.
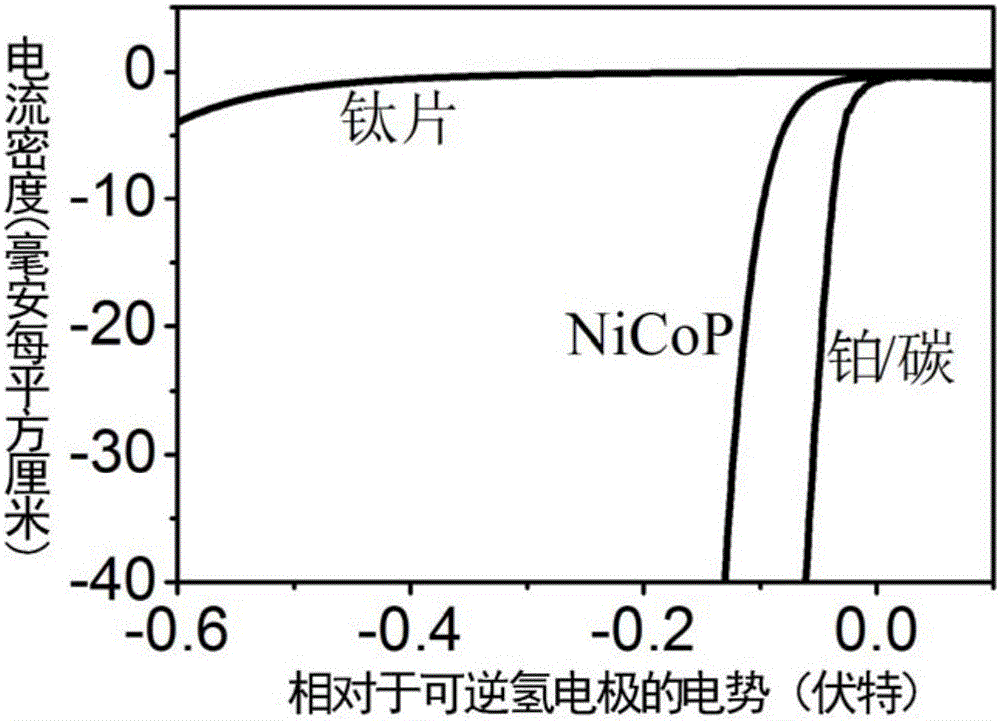
[0070] By testing the catalytic performance of the nickel, cobalt and phosphorus crystals prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com