Novel cephalosporin compound and preparation method thereof
A technology of cephalosporins and compounds, applied in the field of medicine, can solve the problems of low yield, high stability and low impurity content of the preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
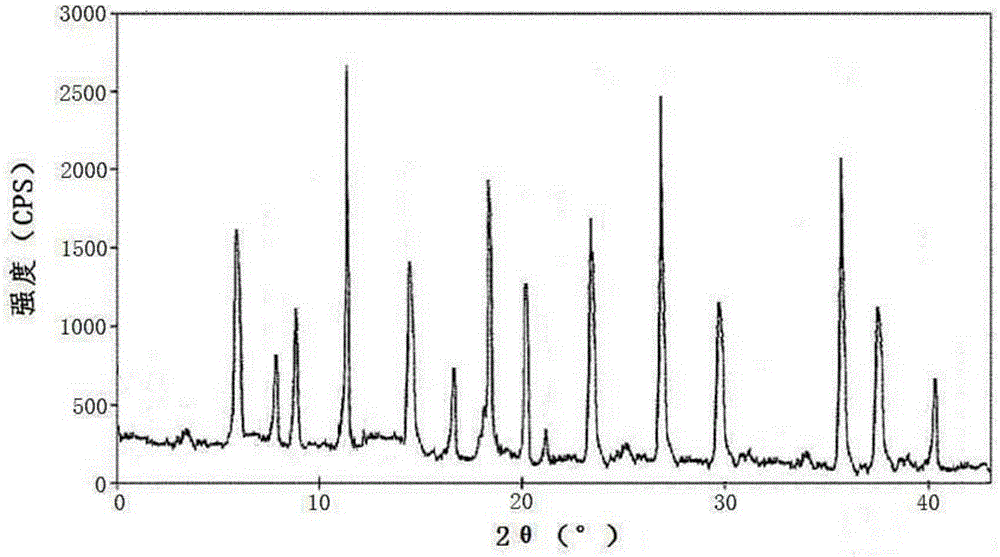
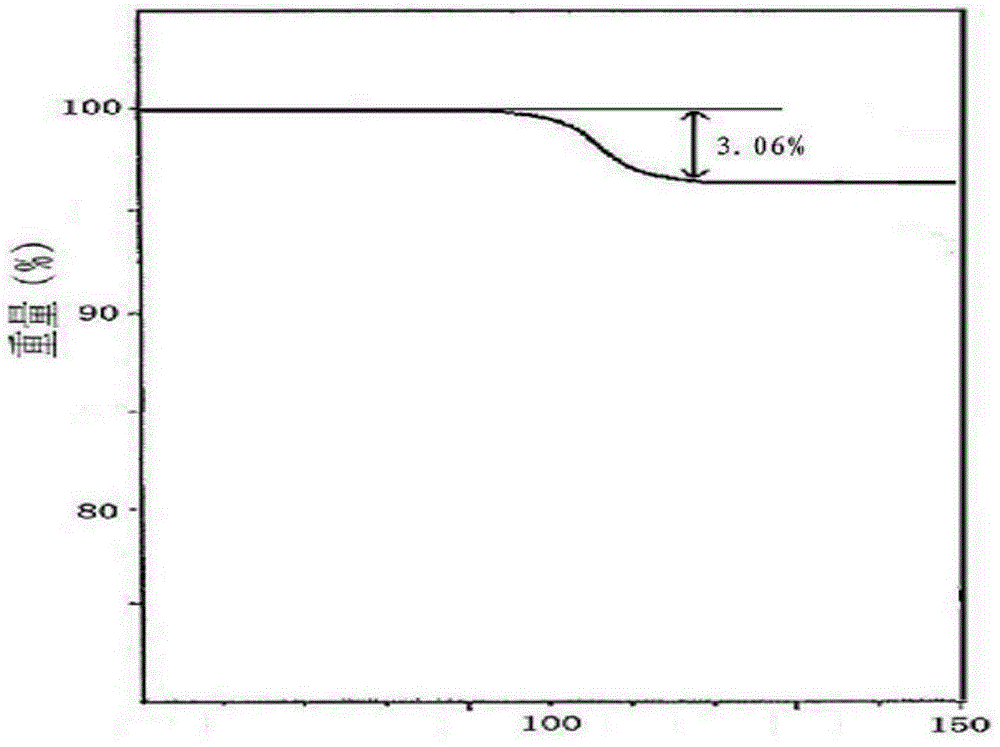
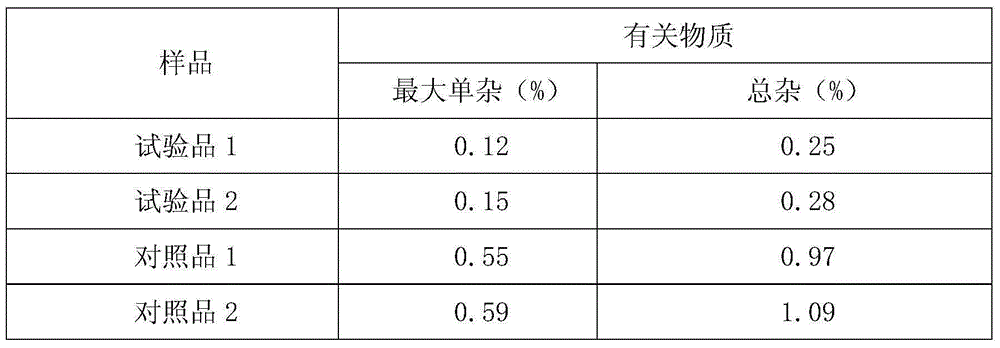
[0033] Example 1: Preparation of Cefoxidone Sodium Crystalline Compound
[0034] 1) Take 1kg of cefoxidone sodium crude product, add it to 15L of mixed solution A at 30~35°C, and stir at a speed of 65r / min to dissolve all of it to obtain cefoxidone sodium solution; wherein the mixed solution A is It is prepared from isopropanol and water in a volume ratio of 1:6;
[0035] 2) Naturally cooling the cefoxidone sodium solution obtained in step 1) to 15~20°C, then adding mixed solvent B at a speed of 15L / min under stirring at a stirring speed of 25r / min, after adding, cooling to 10°C , keep the stirring speed, and grow the crystal for 3 hours;
[0036] The mixed solution B is prepared by acetone and water in a volume ratio of 1:3; the volume ratio of the mixed solution B to the cefoxidone sodium solution is 20:1.
[0037] 3) Cool to 5°C, keep stirring at a stirring speed for 1 hour; keep the temperature at 0~5°C, stand for 2 hours to crystallize, filter, wash the filter cake with...
Embodiment 2
[0042] Example 2: Preparation of Cefoxidone Sodium Crystal Compound
[0043] 1) Take 1kg of cefoxidone sodium crude product, add it to 10L mixed solution A at 30~35°C, and stir at a speed of 55r / min to make all dissolved, to obtain cefoxidone sodium solution, wherein the mixed solution A is It is prepared from isopropanol and water in a volume ratio of 1:4;
[0044] 2) Naturally cool the cefoxidone sodium solution obtained in step 1) to 15-20 °C, then add mixed solvent B at a speed of 10 L / min under stirring at a stirring speed of 15 r / min, and after adding, cool down to 10 °C , keep the stirring speed, and grow the crystal for 2 hours;
[0045] The mixed solution B is prepared by acetone and water in a volume ratio of 1:5; the volume ratio of the mixed solution B and the cefoxidone sodium solution is 15:1.
[0046] 3) Cool to 5°C, keep stirring at a stirring speed for 0.5 hours; keep the temperature at 0~5°C, stand for 4 hours to crystallize, filter, wash the filter cake wi...
Embodiment 3
[0050] Example 3: Preparation of Cefoxidone Sodium Crystalline Compound
[0051] 1) Take 1 kg of cefoxidone sodium crude product, add it to 13 L of mixed solution A at 30~35°C, stir at a speed of 60 r / min to dissolve all of it, and obtain a cefoxidone sodium solution; wherein the mixed solution A is It is prepared from isopropanol and water in a volume ratio of 1:5;
[0052] 2) Naturally cooling the cefoxidone sodium solution obtained in step 1) to 15~20°C, then adding mixed solvent B at a speed of 12L / min under stirring at a stirring speed of 23r / min, after adding, cooling to 10°C , keep the stirring speed, and grow the crystal for 2 hours;
[0053] The mixed solution B is prepared by acetone and water in a volume ratio of 1:4; the volume ratio of the mixed solution B to the cefoxidone sodium solution is 18:1.
[0054] 3) Cool to 5°C, keep stirring at a stirring speed for 1 hour; keep the temperature at 0~5°C, stand for 2 hours to crystallize, filter, wash the filter cake w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com