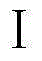Bisindole compound as well as preparation method and application thereof
A bisindole compound and compound technology, applied in the field of bisindole compound and its preparation, achieves the effects of low IC50 concentration, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The strain FG216 was inoculated into a 500 mL Erlenmeyer flask containing 100 mL of seed medium, and the flask was shaken for 3 days at 25°C and 180 r / min. The seed culture liquid was inoculated into the fermentation medium at 1% (v / v), and the flask was shaken for 7 days under the culture conditions of 25°C and 180 r / min. The mycelium of the fermentation broth was separated and extracted with methanol. The methanol extract was used as the fermentation product of FG216. The elution peak at the retention time of 15.6 minutes in high performance liquid chromatography was the bisindole compound (I).
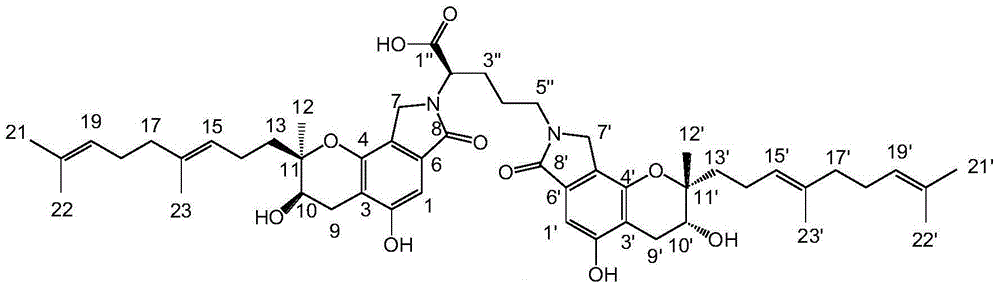
[0033] The structural formula of the bisindole compound (I) is:
[0034]
[0035] The physical and chemical properties of compound (I) are that the appearance is brown powder, and the molecular formula is C. 51 H 68 N 2 O 10 、FAB-MS:869.3867(M+1) + 、ESI-MS:869.3659(M+1) + , ExactMS: 868.4874, melting point 203-205℃, IR: 3397Cm -1 , 2073Cm -1 , 1670Cm -1 , 1465Cm ...
Embodiment 2
[0039] The strain FG216 was inoculated in a 1000 mL Erlenmeyer flask containing 150 mL of seed medium, and the flask was shaken for 3 days under the culture conditions of 20°C and 160 r / min. The seed culture solution was inoculated into the fermentation medium in an amount of 3% (v / v), and the flask was shaken for 10 days under the culture conditions of 25° C. and 180 r / min. The mycelium of the fermentation culture liquid was separated and extracted with ethyl acetate. The ethyl acetate extract was used as the fermentation product of FG216, and the elution peak at the high performance liquid chromatography retention time of 15.6 minutes was the bisindole compound (I).
[0040] Test Data:
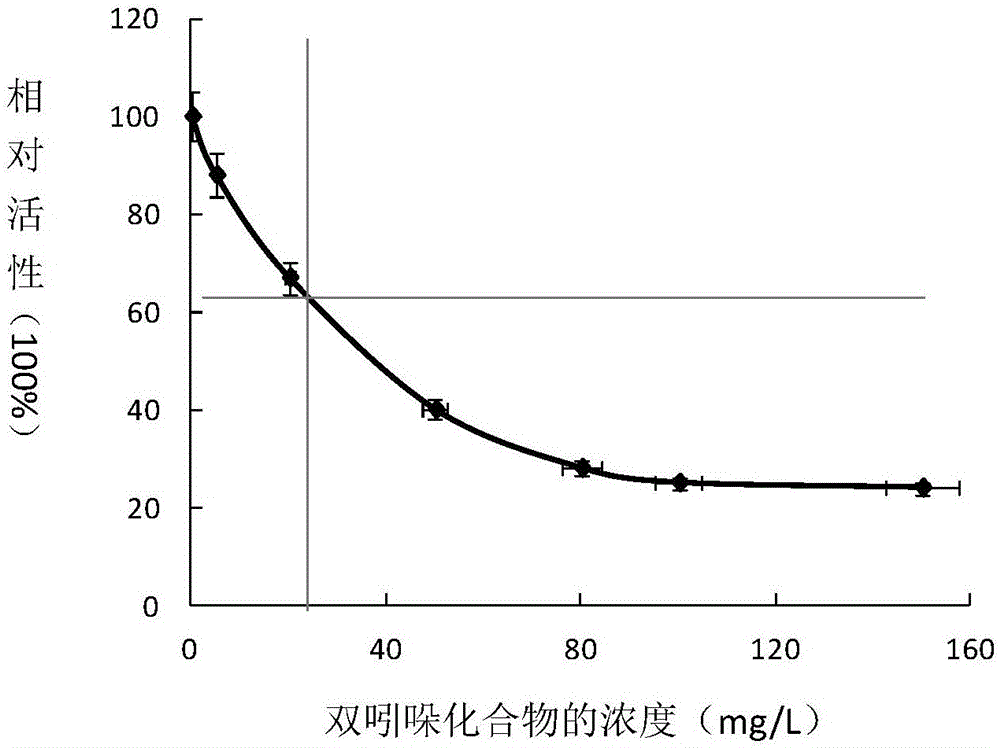
[0041] 3.0mmol / L HMG-CoA, 5U / mgHMG-CoA reductase, 15mmol / LNADPH and the sample to be tested (the bisindole compound prepared in Example 2) were injected into the wells of the 96-well round-bottom plate to form a 100 μL reaction system. The change of NADPH absorbance was continuously measure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com