Photoresponsive cyclometalated gold (III) hydride, preparation method and applications thereof
A ring metal, photoresponsive technology, applied in the field of medicine and chemical industry, can solve the problems of reducing efficiency and selectivity, and achieve good selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 compound I-1
[0042] The specific preparation process of compound Ⅰ-1 is as follows:
[0043]
[0044] (1) Reflux the mixture of ligand 1-2 (1~2g) and mercuric acetate (1.5 equivalents) in absolute ethanol (30mL) for 48 hours, then cool to room temperature, dropwise add chloroform chloride dissolved in hot ethanol Lithium; the white suspension was refluxed for an additional 2 h; after cooling to room temperature, the precipitate was filtered and redissolved in dichloromethane; after removal of insoluble impurities, the solvent of the filtrate was removed under reduced pressure and the crude product was used without further purification 1-3.
[0045] (2) the crude product 1-3 and KAuCl 4 (1 eq.) of the mixture was refluxed in acetonitrile (40 mL) for 48 hours. After cooling to room temperature, a pale yellow / white precipitate was filtered, washed several times with acetonitrile and water, then washed with ether and air-dried to give ...
Embodiment 2
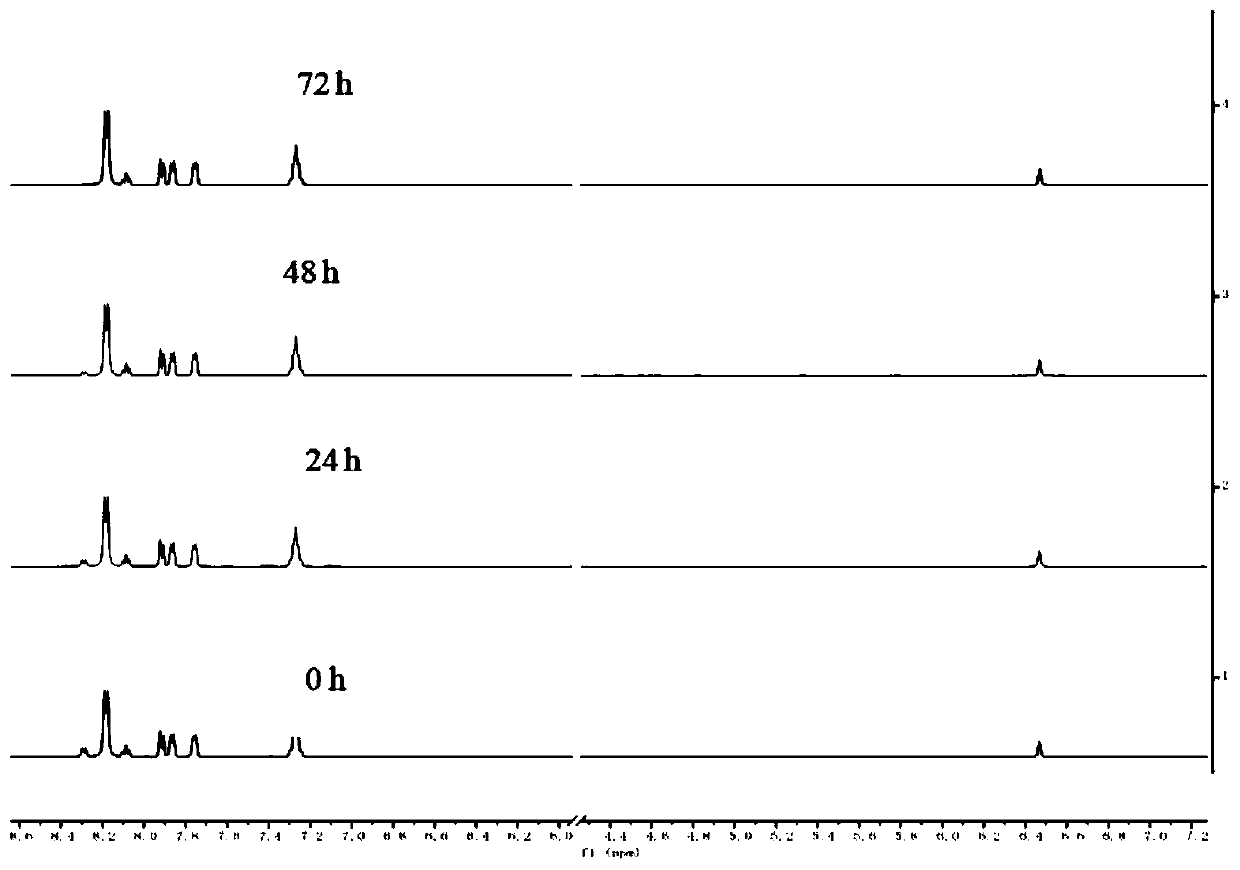
[0052] Test the reaction of the compound with sulfhydryl groups. Taking compound I-1 as an example, test whether it reacts with N-acetylcysteine (NAC) under dark and light conditions. The specific test process is: 100μM compound I-1 with 10mM N-acetylcysteine (NAC) in DMSO-d 6 Mix in medium and place it in the dark for 72 hours. During the standing process, samples were taken every 24 hours for nuclear magnetic detection; after 72 hours, they were irradiated at 420nm, starting from the light, until 5 minutes, 10 minutes, 20 minutes and 30 minutes of light, and the samples were tested by LC. track.
[0053] The NMR test results under dark conditions are as follows: figure 1 As shown, the LC tracking results after illumination are as follows figure 2 shown.
[0054] from figure 1 It can be seen from the figure that after compound I-1 was mixed with N-acetylcysteine (NAC), the nuclear magnetic spectrum did not change within 72 hours, indicating that no reaction occurre...
Embodiment 3
[0057] Taking compound I-2 as an example, test its inhibitory activity on purified TrxR enzyme, the test process is as follows:
[0058] Recombinant human TrxR1 (ICMO Corp, Sweden; 20 nM) was reduced with NADPH (0.2 mM), and then the compound to be tested (i.e. compound I-2) (0-100 nM ), [C^N^C-Au-PPh 3 ]OTf (0-100nM) or C^N^C-Au-NHC(n-Bu)OTf (0-100nM) was added to 2μL TrxR (0.92nM), and the experimental group that did not need light was incubated for 1h and then directly added 5,5 '-Dithiobis(2-nitrobenzoic acid) (DTNB, final 3mM) initiated the reaction and then carried out the determination of OD410nm, the TrxR activity was calculated by the increase rate of the initial OD410nm; for the experimental group requiring light, After incubation for 1 hour and 5 minutes of light, 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB, final 3 mM) was added to initiate the reaction, and then the OD 410 nm was measured. where [C^N^C-Au-PPh 3 ]OTf is a positive control, and C^N^C-Au-NHC(n-Bu)OT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com