Method for cultivating lymph-node autologous CIK (cytokine-induced killer) cells and application of lymph-node autologous CIK cells
A lymph node and cell technology, applied in the fields of biotechnology and microbial animal cell lines, can solve the problems of no obvious improvement in the overall survival rate of lung cancer patients, difficult to culture CIK cells, serious toxicity, etc., and achieve strong anti-tumor proliferation ability in vitro and no tumor. Controlling cell contamination and preventing recurrence and metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058]1) Induction and expansion of CIK cells in lymph nodes and peripheral blood CIK cells
[0059] (1) 50 mL of isolated venous blood from lung cancer patients was stored at 16-20°C, and the peripheral blood was diluted by Ficoll density gradient centrifugation (density 1,077 g / mL): 50 mL of peripheral blood + 40 mL of normal saline Blood samples were separated and centrifuged by Ficoll (2000 rpm, 670 (×g) for 20 minutes), and the misty peripheral blood mononuclear cell layer in the middle layer was taken and left in the incubator for 2 hours, and then the suspension cells were used to prepare CIK;
[0060] (2) About 10 g of isolated mediastinal lymph node tissue from lung cancer patients (paraffin section staining and pathology confirmed no metastasis), stored at 16-20°C, shredded, removed adipose tissue and various fiber components, and then ground, After washing and resuspending in 0.9% sodium chloride solution, the cells were collected by filtering with a cell strainer. ...
Embodiment 2
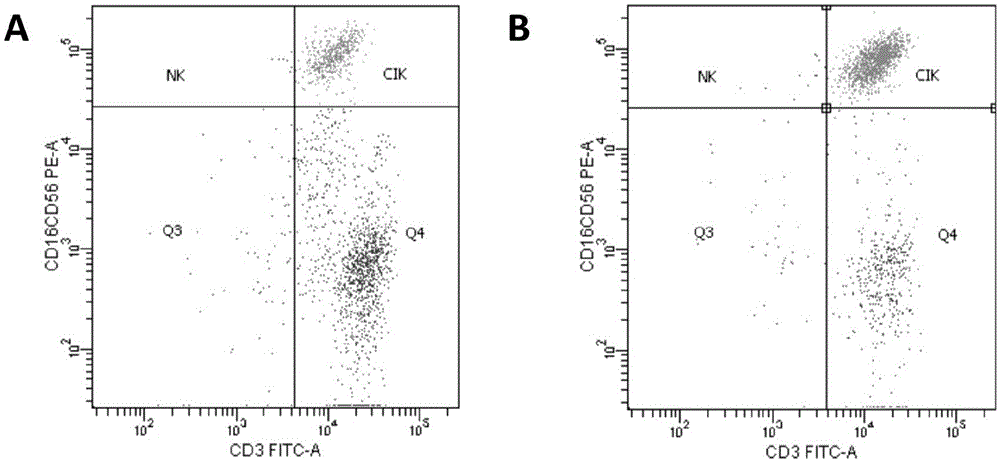
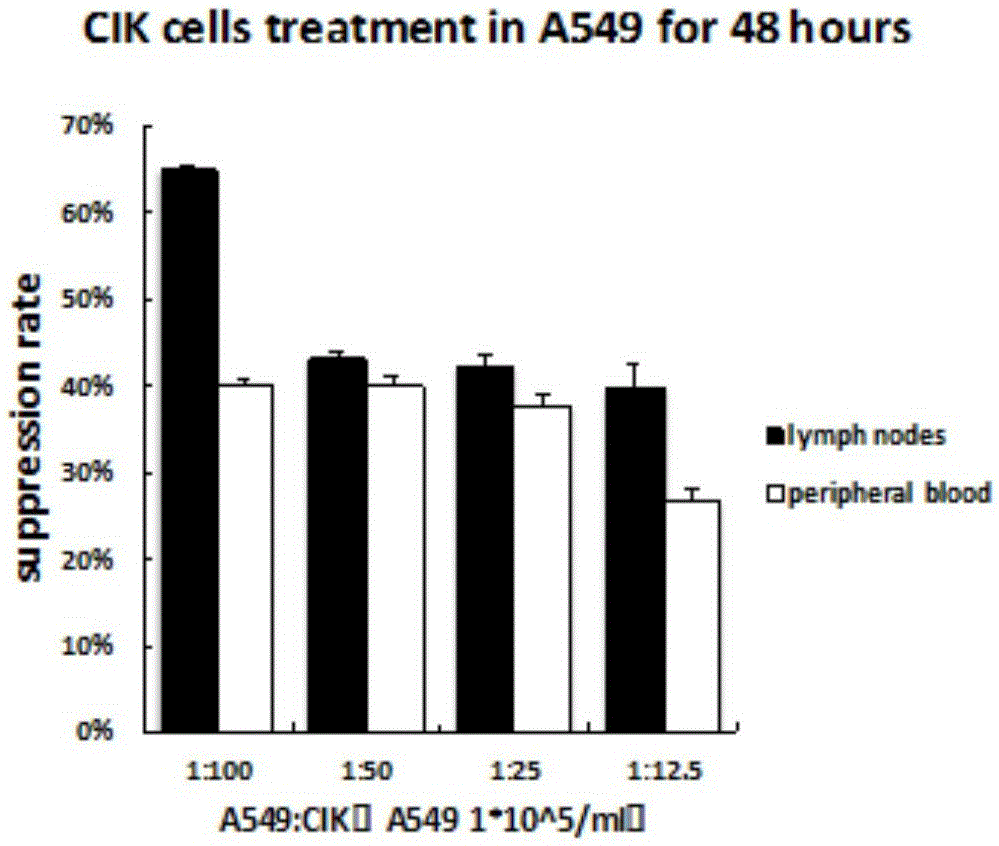
[0073] Example 2 Antiproliferation and Cytotoxicity Tests on A549 Cells
[0074] Peripheral blood obtained before operation and lymph nodes obtained during operation were cultured for 14 days to obtain CIK cells from each patient according to the method 1.3.2, and reinfused continuously for 4 days starting from the 15th day, (lymph node group d1+peripheral blood group d2) alternately 2 reinfusions; peripheral blood was drawn on the 14th day and 7 days after the reinfusion to detect carcinoembryonic antigen (CEA), blood routine (to assess the incidence of bone marrow suppression), and to observe the incidence of rash, fever, and allergic events;
[0075] The SPSS11.0 software was used, and the data were represented by x±s; the comparison between the inhibition rates detected by the CCK8 method was performed by the t test, and the difference was statistically significant when P<0.05;
[0076] The results of the anti-proliferation and cytotoxic effects of lymph node CIK cells and...
Embodiment 3
[0079] Embodiment 3 clinical intervention experiment
[0080] For the unbalanced immune system of lung cancer patients, the intervention strategies of activated killer T cells combined with dendritic cells (CIK-DC) cultured and activated from autologous lymph nodes of patients with lung cancer surgery, combined with chemotherapy can inhibit the recurrence and metastasis of lung cancer patients and improve the patient's resistance. Cancer immunity, thereby improving the disease-free survival and overall survival of lung cancer patients.
[0081] According to the point of view of immune staging of lung cancer, the number of CD8+T lymphocytes and FOX-P3+T lymphocytes corresponds to the traditional pathological stage, which represents tumor size T; CD45RO+ memory T cells corresponds to the traditional pathological stage, which represents distant metastasis; M; Thus, the traditional pathological TNM staging of lung cancer is leapfrogged to the TNM staging representing the body's an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com