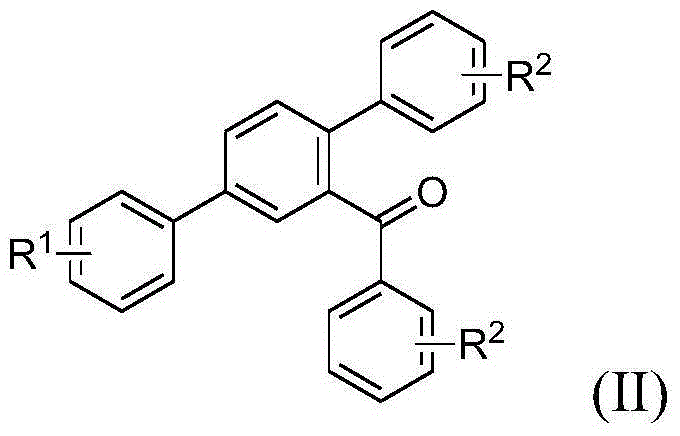
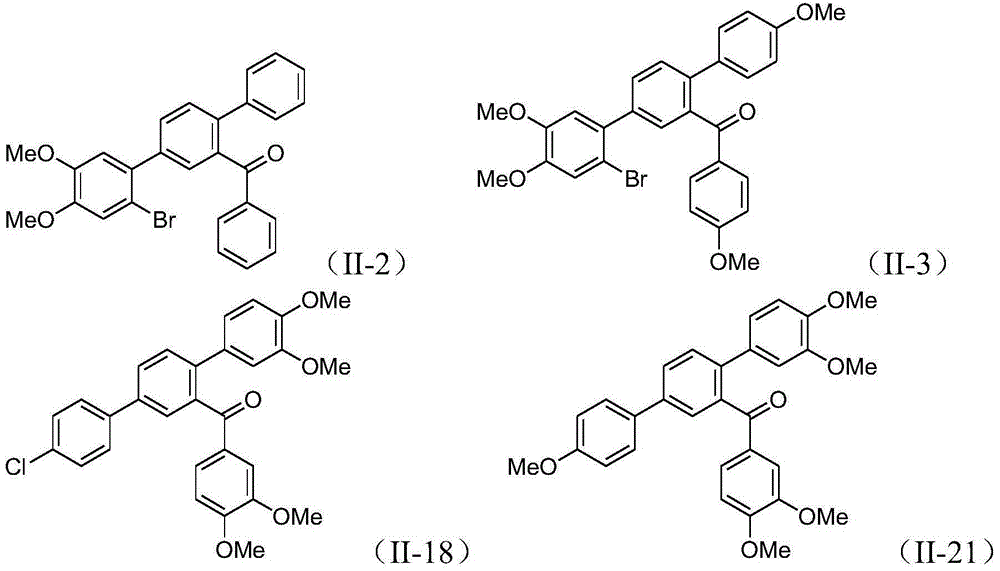
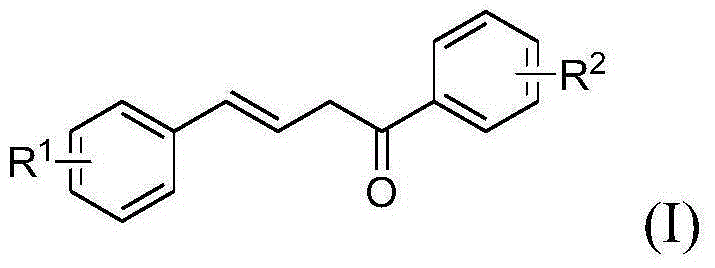
Terphenyl compound and preparation method therefor and application thereof
A technology of ketone compounds and compounds, which is applied in the field of terphenyl compounds and their preparation and application, can solve the problems of precious metal catalysts and use prices, and achieve the effects of single product structure, convenient operation, and good practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] Preparation of compound (I-3)
[0045]Add 0.5635g (2.34mmol) 3,4-dimethoxy-6-bromophenylacetylene, 0.4670g (3.11mmol) p-methoxyacetophenone, 0.3570g (3.18mmol) t-BuOK to the test tube reactor , 5 ml DMSO. Heated in an oil bath at 100°C for 1.5h. After the reaction, add water, extract with dichloromethane, combine the organic layers, and concentrate the residue to separate by column chromatography (eluent is petroleum ether: ethyl acetate = 10: 1), 0.2844g of the target compound was obtained, with a yield of 31%. M.p.133-134℃
[0046] Preparation of compound (I-4)
[0047] Add 0.2101g (2.06mmol) phenylacetylene, 0.3123g (2.08mmol) p-methoxyacetophenone, 0.2467g (2.20mmol) t-BuOK, 5mlDMSO into the test tube reactor. Heated in an oil bath at 100°C for 1.5h. After the reaction, add water, extract with dichloromethane, combine the organic layers, and concentrate the residue to separate by column chromatography (eluent is petroleum ether: ethyl acetate = 10: 1), 0.3272g ...
Embodiment 1
[0080] Embodiment 1: the preparation of compound (II-1)
[0081] Add β, γ-unsaturated ketone (I-1) (0.1840g, 0.7293mmol) and cesium carbonate (0.2796g, 0.7967mmol) into the reaction vessel, mix in 1,4-dioxane (2mL), Stir and react in an oil bath at 90°C for 13 hours; after the reaction, add water, extract with dichloromethane, combine the organic layers, and concentrate the residue to separate by column chromatography (eluent is petroleum ether: dichloromethane: = 1: 1, V:V), collect R f The eluate with a value of 0.3-0.35 was distilled under reduced pressure and dried to obtain the target compound (II-1), 0.0821 g, with a yield of 61.8%.
[0082] 1 HNMR (500MHz, CDCl 3 )δ7.79(dd, J=8.0,1.9Hz,1H),7.73-7.72(m,3H),7.62(d,J=8.8Hz,2H),7.56(d,J=8.0Hz,1H), 7.43(t, J=7.4Hz1H), 7.33-7.29(m, 4H), 7.23(t, J=7.4Hz, 2H), 7.19-7.16(m, 1H), 7.02(d, J=8.8Hz, 2H ),3.88(s,3H)
[0083]
Embodiment 2
[0085] The cesium carbonate was changed to potassium tert-butoxide (0.0831g, 0.7405mmol), and other operations were the same as in Example 1 to obtain the target compound (II-1), 0.0774g, with a yield of 58.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com