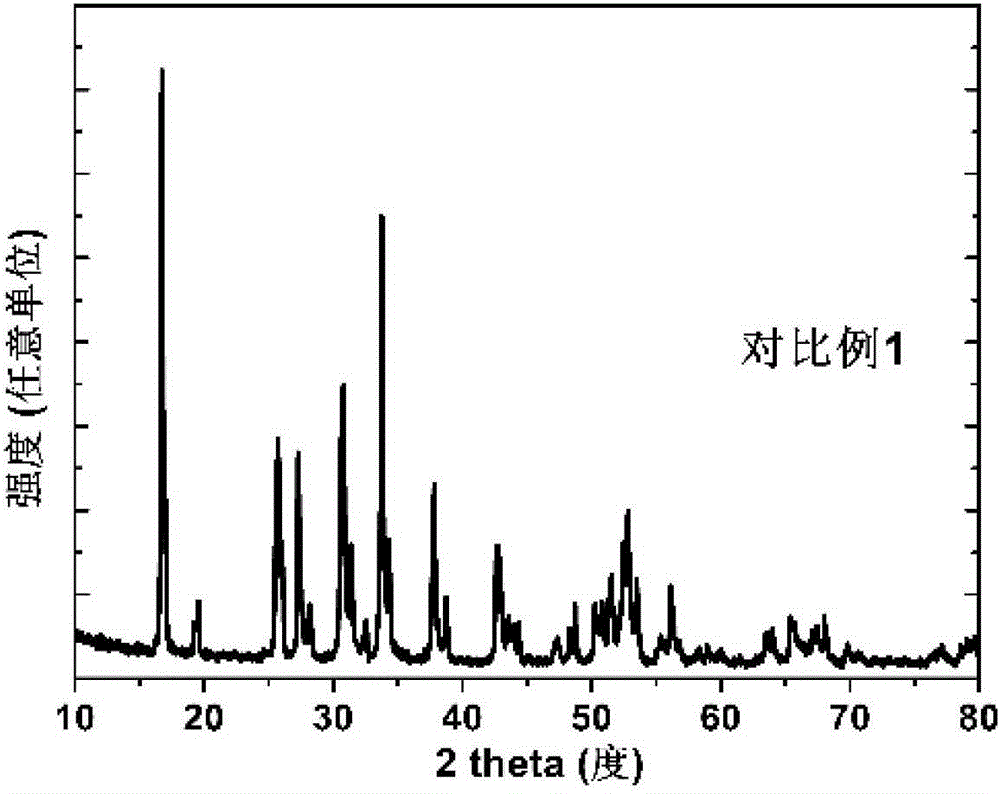
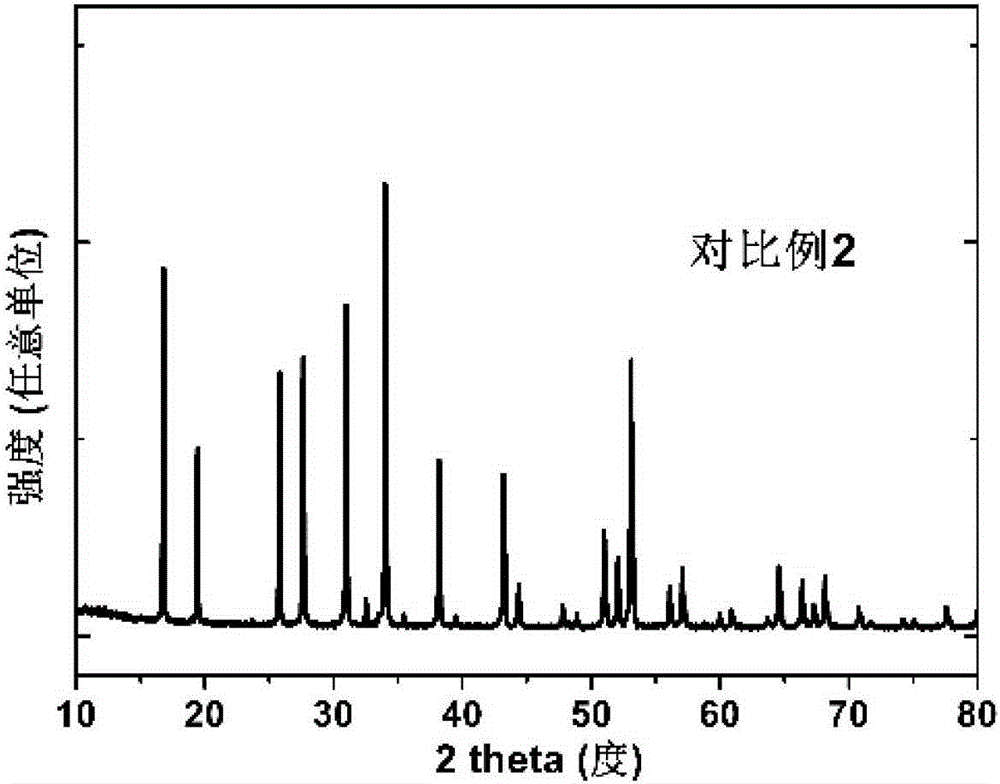
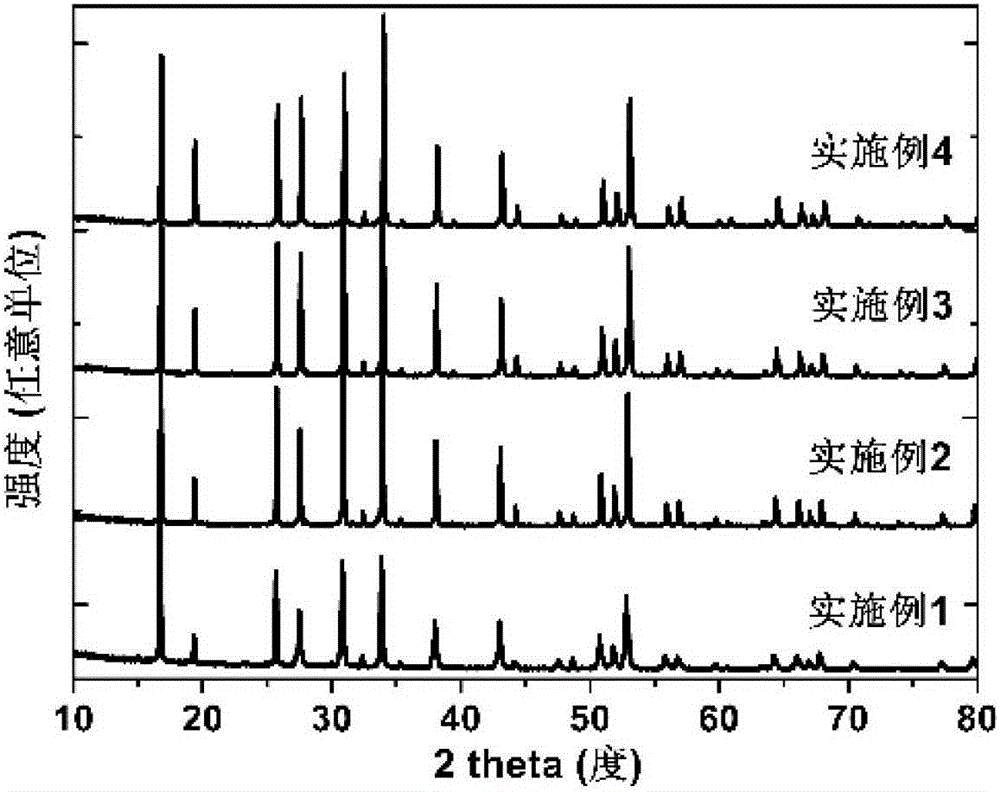
Garnet-structured ceramic electrolyte material, preparation method and application therefor
A ceramic electrolyte and garnet technology, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of difficult accurate control of lithium content, complex synthesis process, and reduction of ceramic density and ion conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The chemical composition is 94wt% Li prepared by high-temperature solid-phase method 7.50 La 3.00 Zr 1.75 W 0.25 o 12.50 +6wt%Al 2 o 3 The ceramic electrolyte: LiOH·H 2 O powder, La(OH) 3 Powder, ZrO 2 Powder and WO 3 The powder was weighed according to the stoichiometric ratio, dried in an alcoholic medium for 12 hours, then calcined at 900°C for 12 hours and cooled to room temperature at a heating rate of 3°C / min and a cooling rate of 2°C / min to obtain Li 7.50 La 3 Zr 1.75 W 0.25 o 12.50 Ceramic powder. Then with 94wt% Li 7.50 La 3 Zr 1.75 W 0.25 o 12.50 +6wt%Al 2 o 3 As a ceramic component, add 6wt.% Al to the ceramic powder 2 o 3 The powder is ball milled in an alcohol medium for 24 hours and then dried to obtain the mother powder. Weigh a certain amount of mother powder and dry press it under a pressure of 200Mpa, then put the molded body into an alumina crucible with a lid, surround the molded body with mother powder, raise the temperature t...
Embodiment 2
[0041] The chemical composition is 96wt% Li prepared by high-temperature solid-state method 7.30 La 3.00 Zr 1.65 W 0.35 o 12.50 +4wt%Y 2 o 3 The ceramic electrolyte: LiOH·H 2 O powder, La(OH) 3 Powder, ZrO 2 Powder and WO 3 The powder was weighed according to the stoichiometric ratio, dried in an alcoholic medium for 12 hours, then calcined at 900°C for 12 hours and cooled to room temperature at a heating rate of 3°C / min and a cooling rate of 2°C / min to obtain Li 7.30 La 3.00 Zr 1.65 W 0.35 o 12.50 Ceramic powder. Then with 96wt% Li 7.30 La 3.00 Zr 1.65 W 0.35 o 12.50 +4wt%Y 2 o 3 As a ceramic component, add 4wt.% of Y to the ceramic powder 2 o 3 The powder is ball milled in an alcohol medium for 24 hours and then dried to obtain the mother powder. Weigh a certain amount of mother powder and dry press it under a pressure of 200Mpa, then put the molded body into an alumina crucible with a lid, surround the molded body with mother powder, raise the tempera...
Embodiment 3
[0043] The chemical composition is 97wt% Li prepared by high-temperature solid-phase method 7.10 La 3.00 Zr 1.65 W 0.45 o 12.50 +3wt%B 2 o 3 The ceramic electrolyte: LiOH·H 2 O powder, La(OH) 3 Powder, ZrO 2 Powder and WO 3 The powder was weighed according to the stoichiometric ratio, dried in an alcoholic medium for 12 hours, then calcined at 900°C for 12 hours and cooled to room temperature at a heating rate of 3°C / min and a cooling rate of 2°C / min to obtain Li 7.10 La 3.00 Zr 1.65 W 0.45 o 12.50 Ceramic powder. Then with 97wt% Li 7.10 La 3.00 Zr 1.65 W 0.45 o 12.50 +3wt%B 2 o 3 As a ceramic component, add 3wt.% B in the ceramic powder 2 o 3 The powder is ball milled in an alcohol medium for 24 hours and then dried to obtain the mother powder. Weigh a certain amount of mother powder and dry press it under a pressure of 200Mpa, then put the molded body into an alumina crucible with a lid, surround the molded body with mother powder, raise the temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com