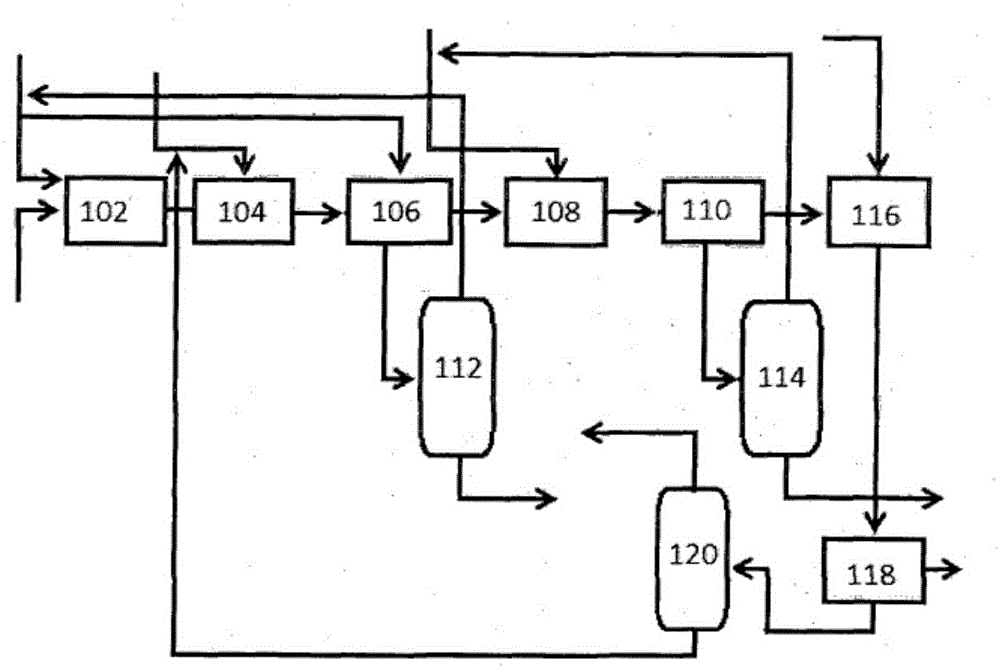
A process for regenerating ionic compound
A technology of ionic compounds and chlorides, applied in separation methods, hydrocarbons, hydrocarbons, etc., can solve problems such as low energy efficiency, inability to reuse/recycle, and major equipment expenditures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] In this experiment, chloroaluminate-based ionic compounds were prepared, deactivated by repeated use in alkylation reactions, and then the deactivated ionic compounds were regenerated by the disclosed method.
[0072] Step i: Preparation of chloroaluminate ionic compound from 1-butyl-3-methylimidazolium bromide [BMIM]Br
[0073] The apparatus consisted of a 5 L three necked round bottom flask equipped with an overhead stirrer and placed in an ice bath at 0-5 °C. The flask was clamped to provide stability while stirring. Keep the whole assembly under nitrogen atmosphere. Weigh 680 g of [BMIM]Br and carefully fill the flask through the funnel. Weigh 830gAlCl 3 , and slowly added to the flask under constant stirring. AlCl 3 The loading of is completed within 1.5 hours. The mixture was further stirred for 2 hours to properly mix the ingredients. The final catalyst was kept under nitrogen.
[0074] Step ii: Inactivation of the ionic compound prepared in step (i) by r...
Embodiment 2
[0080] Example 2: Recovery of [BMIM]Br from fresh catalyst by neutralization with triethylamine
[0081]In this experiment, the ionic compound prepared in step (i) of Experiment 1 was directly regenerated without deactivation to investigate the efficiency of the disclosed method.
[0082] 10.0 g of fresh catalyst obtained from step (i) of Experiment 1 were charged together with 50 ml of ethyl acetate into a 250 ml round bottom flask equipped with an overhead stirrer maintained at 29°C. 12.6 g of triethylamine were slowly added over a period of 1 hour and stirred for 30 minutes. The resulting mixture was filtered and ethyl acetate was separated. The resulting solid was washed in batches with 200 ml of dichloromethane to extract [BMIM]Br from the solid mixture. The dichloromethane layer thus obtained was distilled off to obtain 4 g of [BMIM]Br salt. The yield obtained was 88%.
Embodiment 3
[0083] Example 3: Regeneration of the Ionic Compound [BMIM]Br Using Sodium Carbonate Using the Disclosed Method
[0084] The method used in this experiment was the same as that described in step (iii) of Experiment 1, except that sodium carbonate solution was used instead of triethylamine.
[0085] 100 g of the deactivated catalyst obtained in step (ii) of Experiment 1 was charged into a 500 ml Erlenmeyer flask. By mixing 133.3gNa 2 CO 3 and 350ml of water to prepare Na 2 CO 3 solution, and set aside. Na will be produced 2 CO 3 The solution was slowly added to the Erlenmeyer flask containing the deactivated catalyst. Once the addition was complete, 100 ml of ethyl acetate was added to the flask and stirred for 1 hour and allowed to stand for 1 hour. The water and ethyl acetate layers were separated, and the aqueous layer was subjected to distillation to obtain a solid mixture. The solid mixture was washed with 75 ml of dichloromethane to extract [BMIM]Br, and the resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com