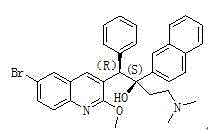
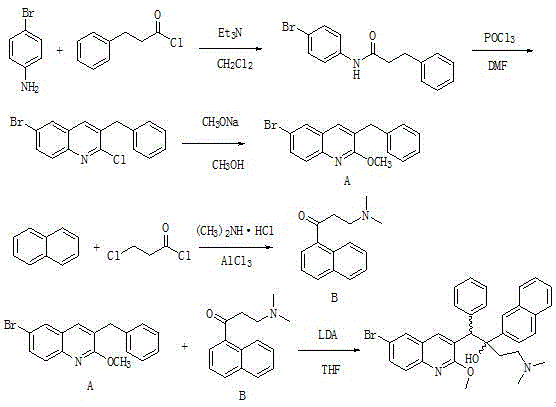
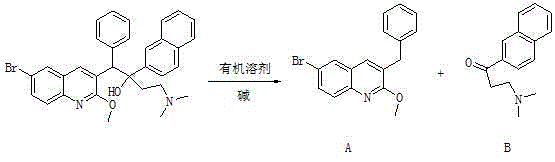
Method for high efficiency production of Bedaquiline
A quinoline-based, product-based technology, applied in the field of efficient production of bedaquiline, can solve the problems of material waste, no recycling of the three isomers, and unsuitability for large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Add 0.1kg of 1-(6-bromo-2-methoxy-3-quinolyl)-4-dimethylamino-2-(1-naphthyl)-1-phenyl-2-butanol to 2L In tetrahydrofuran, after stirring, 0.1 kg of sodium hydroxide was added, the reaction was stirred at room temperature, dried over anhydrous sodium sulfate, and spin-dried to obtain the crude product mixture compound A and compound B.
[0040] The resulting crude product mixture was dissolved in ethyl acetate, hydrochloric acid was added, and the organic layer was spin-dried to obtain 51.06 g of compound A, with a yield of 86.4% and a purity of 97.8%; After ester extraction, the organic layers were combined and dried over anhydrous sodium sulfate to obtain 33.82 g of compound B with a yield of 82.7% and a purity of 94.5%.
Embodiment 2
[0042] Add 0.1kg of 1-(6-bromo-2-methoxy-3-quinolinyl)-4-dimethylamino-2-(1-naphthyl)-1-phenyl-2-butanol to 1L In dichloromethane, after stirring, 0.45 kg of cesium hydroxide was added, stirred at room temperature for reaction, dried over anhydrous sodium sulfate, and spin-dried to obtain crude compound A and compound B.
[0043] The resulting crude product mixture was dissolved in toluene, phosphoric acid was added, and the organic layer was spin-dried to obtain 50.7 g of compound A, with a yield of 85.8% and a purity of 97.3%; the acid layer was adjusted to alkaline with potassium bicarbonate, extracted with toluene, and combined The organic layer was dried over anhydrous sodium sulfate to obtain 33.74 g of compound B with a yield of 82.5% and a purity of 94.4%.
Embodiment 3
[0045] Add 0.1kg of 1-(6-bromo-2-methoxy-3-quinolyl)-4-dimethylamino-2-(1-naphthyl)-1-phenyl-2-butanol to 4L In acetonitrile, 0.15 kg of sodium ethoxide was added after stirring, the reaction was stirred at room temperature, dried over anhydrous sodium sulfate, and spin-dried to obtain the crude product mixture compound A and compound B.
[0046] The resulting crude product mixture was dissolved in dichloromethane, hydrochloric acid was added, and the organic layer was spin-dried to obtain 50.35 g of compound A, with a yield of 85.2% and a purity of 97.6%; Extract, combine the organic layers, and dry over anhydrous sodium sulfate to obtain 33.17 g of compound B, with a yield of 81.1% and a purity of 94.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com