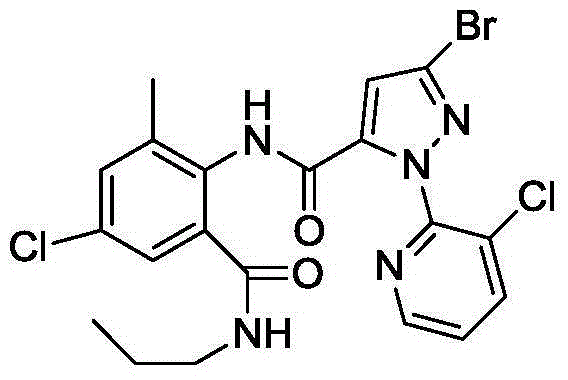
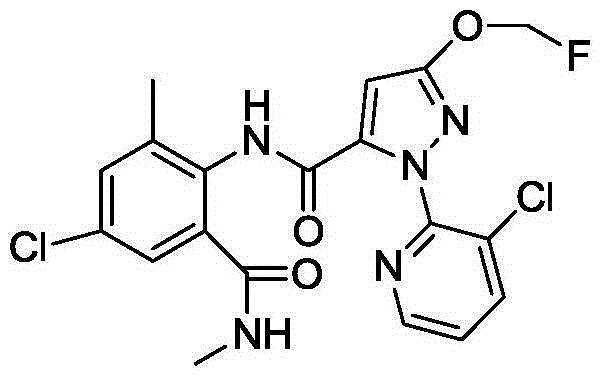
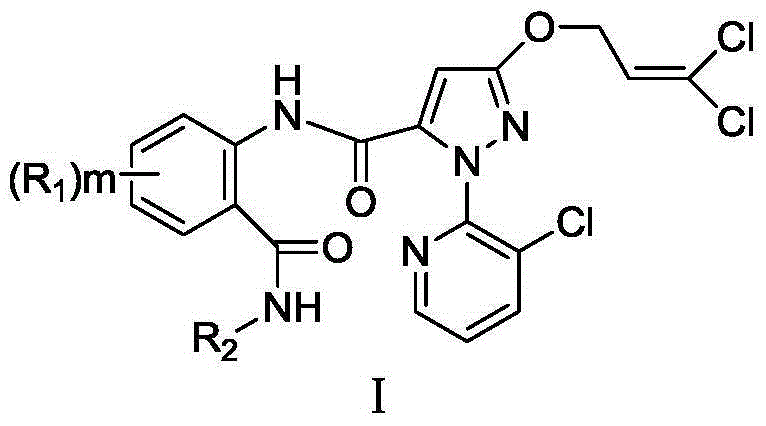
Anthranilic diamide compound containing dichloropropene base and application
A technology of o-formamidobenzamide and dichloropropenyl, applied in the field of o-formamidobenzamide compounds, can solve the problems of reduced productivity and increased consumer costs, achieve high insecticidal activity, and alleviate drug resistance Questions, rich variety of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] N-[2-(methylcarbamoyl)-4-chloro-6-methyl-phenyl]-1-(3-chloro-2-pyridyl)-3-[(3,3-dichloropropene Base) Oxygen]-1H-pyrazole-5-carboxamide Synthesis:
[0042] Step A: Synthesis of 2-amino-5-chloro-3-methylbenzoic acid
[0043] Add 2-amino-3-methylbenzoic acid (5g, 33mmol) and DMF20mL into a 50mL three-necked flask, stir to dissolve, the solution is purple, then add NCS (4.4g, 33mmol), and heat the reaction solution to 100°C for 1h , after the completion of the reaction, cool to room temperature, then slowly pour the reaction solution into ice water and keep stirring (purple red solids are precipitated during the process), after stirring for half an hour, suction filter to obtain purple red solid 2-amino-5-chloro-3 -Methylbenzoic acid, weighing 7g after drying.
[0044] Step B: Synthesis of ethyl 1-(3-chloro-2-pyridyl)-3-hydroxy-1H-pyrazole-5-carboxylate
[0045] Add 1-(3-chloro-2-pyridyl)-3-hydroxy-4,5-dihydro-1H-pyrazole-5-carboxylic acid ethyl ester (11 g, 40.7 mmol) ...
Embodiment 2
[0054] N-[2-(cyclopropylcarbamoyl)-4-chloro-6-methyl-phenyl]-1-(3-chloro-2-pyridyl)-3-[(3,3-dichloro Synthesis of propenyl)oxy]-1H-pyrazole-5-carboxamide:
[0055] In a 100 mL round bottom flask, add 1.0 g of 6-chloro-2-[1-(3-chloro-2-pyridyl)-3-[(3,3-dichloropropenyl)oxy]-1H-5- Pyrazolyl]-8-methyl-4H-[d][1,3]benzoxazin-4-one (the product of step D in Example 1), dissolved in 30 mL of acetonitrile (turbid), then add cyclopropylamine solution , the molar ratio was 1:3, and reacted at 30°C for 4h. After the reaction, the solvent was removed under reduced pressure, then dissolved with ethyl acetate, washed with dilute hydrochloric acid, water and saturated brine, and the organic layer was dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated, and the residue was subjected to silica gel column chromatography, and the eluent was a mixed solvent of ethyl acetate and petroleum ether to obtain the target compound.
Embodiment 3
[0057] N-[2-(carbamoyl)-4-chloro-6-methyl-phenyl]-1-(3-chloro-2-pyridyl)-3-[(3,3-dichloropropenyl) Synthesis of oxy]-1H-pyrazole-5-carboxamide:
[0058] In a 100 mL round bottom flask, add 1.0 g of 6-chloro-2-[1-(3-chloro-2-pyridyl)-3-[(3,3-dichloropropenyl)oxy]-1H-5- Pyrazolyl]-8-methyl-4H-[d][1,3]benzoxazin-4-one (the product of Step D of Example 1), dissolved in 30 mL of acetonitrile (turbid), and then added ammonia solution, The molar ratio was 1:3, and the reaction was carried out at 30°C for 4h. After the reaction, the solvent was removed under reduced pressure, then dissolved with ethyl acetate, washed with dilute hydrochloric acid, water and saturated brine, and the organic layer was dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated, and the residue was subjected to silica gel column chromatography, and the eluent was a mixed solvent of ethyl acetate and petroleum ether to obtain the target compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com