Photodynamically active platinum compound with near-infrared absorption, preparation method and application thereof
A technology of photodynamics and compounds, applied in the direction of organic active ingredients, chemical instruments and methods, compounds containing elements of group 8/9/10/18 of the periodic table, etc., can solve serious drug resistance, toxic and side effects and other problems , to achieve the effects of reducing toxic and side effects, good biocompatibility, and enhancing tumor targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
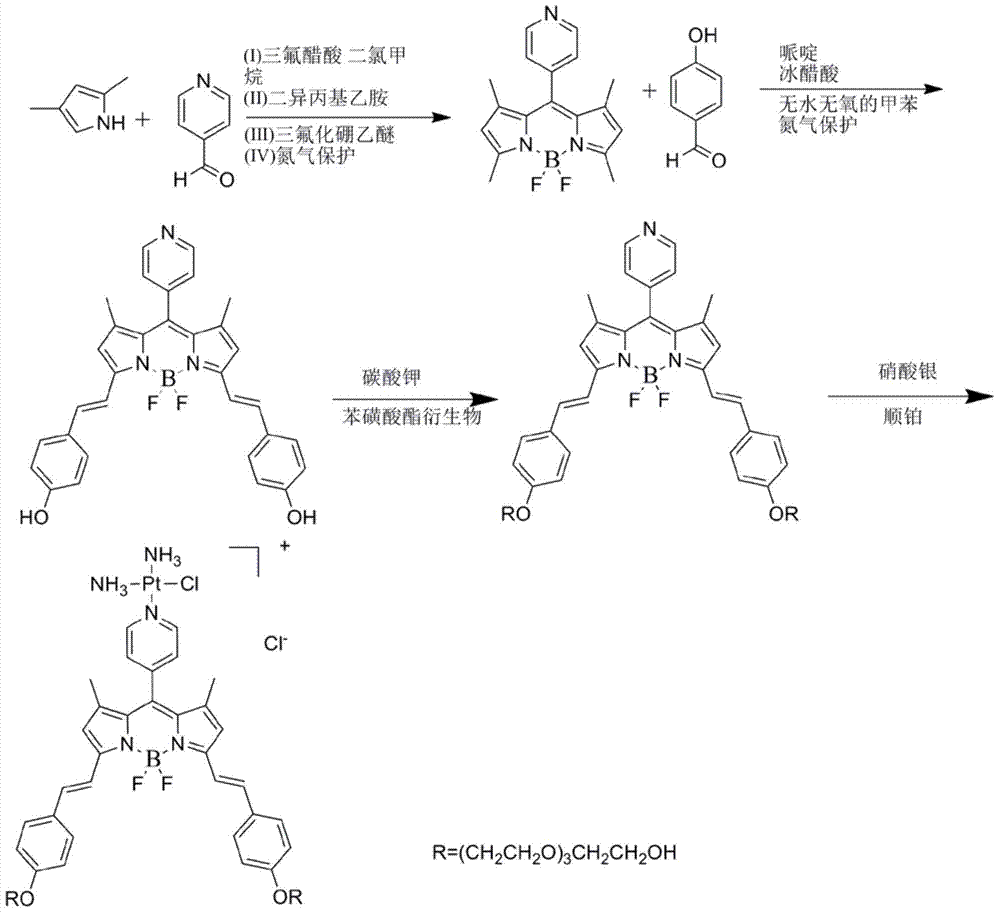
[0041] (1) Preparation of fluoroborate dipyrrole derivatives: according to figure 1 As shown in the first step in the reaction equation, the fluoroboron dipyrrole derivative is prepared by a conventional method.
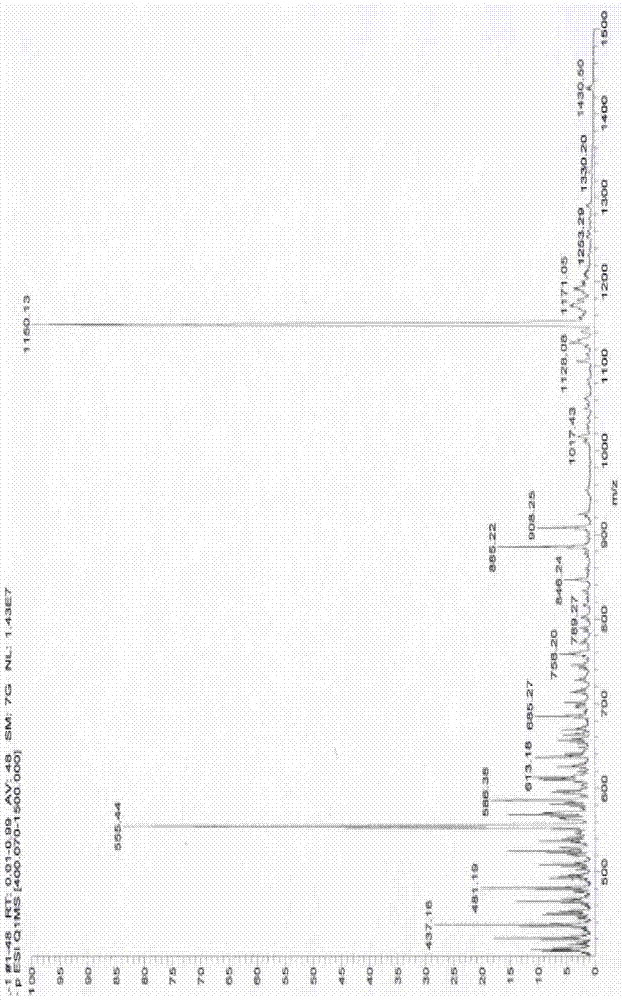
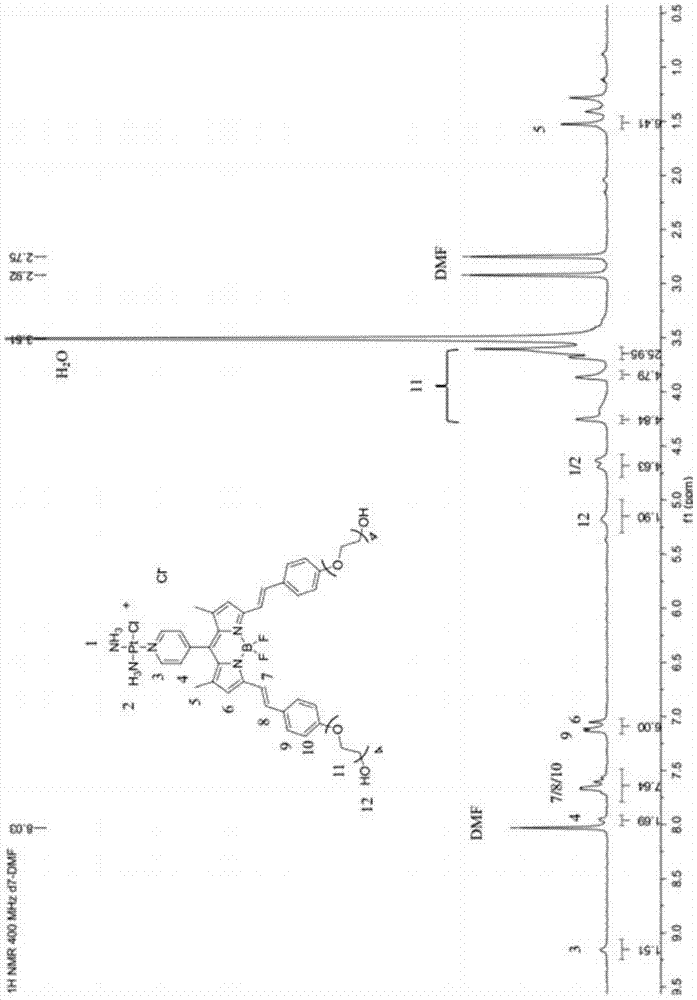
[0042] (2) Preparation of fluoroboron dipyrrole extended conjugate: according to figure 1 As shown in the second step in the reaction equation, weigh 130 mg of fluoroborate dipyrrole derivatives prepared in (1) and 147 mg of p-Hydroxybenzaldehyde dissolved in toluene with a volume ratio of 100-300 times, and add 1 ml of ice Acetic acid and 1 ml of piperidine were heated to reflux under nitrogen atmosphere for 20 hours, and then ice-bathed for 30 minutes. The resulting solid product was washed several times with toluene, and then spin-dried by a rotary evaporator to obtain a crude product. Then, the crude product was separated by silica gel chromatography, and the eluent was acetone-petroleum ether system to obtain the purified fluorobodipyrrole extended conjugate wi...
Embodiment 2
[0046] (1) Preparation of fluoroborate dipyrrole derivatives: according to figure 1 As shown in the first step in the reaction equation, the fluoroboron dipyrrole derivative is prepared by a conventional method.
[0047] (2) Preparation of fluoroboron dipyrrole extended conjugate: according to figure 1 Shown in the second step in the reaction equation, weigh 305 mg of the obtained fluoroborate dipyrrole derivatives prepared in (1) and 420 mg of p-Hydroxybenzaldehyde dissolved in toluene with a volume ratio of 100-300 times, and add 0.1 ml of ice Acetic acid and 1 ml of piperidine were heated to reflux under nitrogen atmosphere for 10 hours, and then ice-bathed for 60 minutes. The resulting solid product was washed several times with toluene, and then spin-dried by a rotary evaporator to obtain a crude product. Then, the crude product was separated by silica gel chromatography, and the eluent was acetone-petroleum ether system to obtain the purified fluorobodipyrrole extended ...
Embodiment 3
[0051] (1) Preparation of fluoroborate dipyrrole derivatives: according to figure 1 As shown in the first step in the reaction equation, the fluoroboron dipyrrole derivative is prepared by a conventional method.
[0052] (2) Preparation of fluoroboron dipyrrole extended conjugate: according to figure 1 As shown in the second step in the reaction equation, weigh 30 mg of the obtained fluoroborate dipyrrole derivative prepared in (1) and 47 mg of p-Hydroxybenzaldehyde dissolved in toluene with a volume ratio of 100-300 times, and add 0.5 ml of ice Acetic acid and 0.5 ml of piperidine were heated to reflux under nitrogen atmosphere for 10 hours, then ice-bathed for 40 minutes, and the resulting solid product was washed several times with toluene, and then spin-dried by a rotary evaporator to obtain a crude product. Then, the crude product was separated by silica gel chromatography, and the eluent was acetone-petroleum ether system to obtain the purified fluorobodipyrrole extende...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com