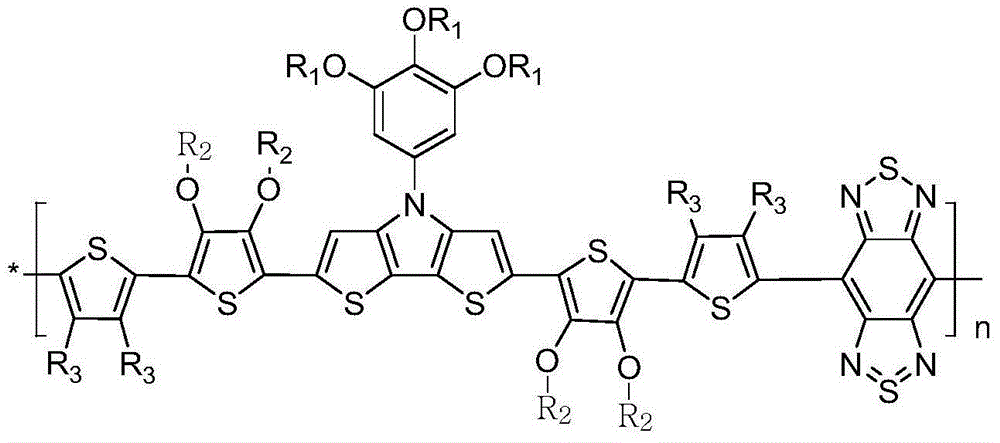
Long-wave absorption copolymer donor material for polymer solar cell light active layer and preparation method of long-wave absorption copolymer donor material
A solar cell and photoactive layer technology, which is applied in the direction of electrical solid-state devices, semiconductor/solid-state device manufacturing, circuits, etc., can solve the problems of low conjugation degree, poor solubility, harsh synthesis conditions, etc., to improve conjugation degree, Effect of high degree of conjugation, enhanced solubility and processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
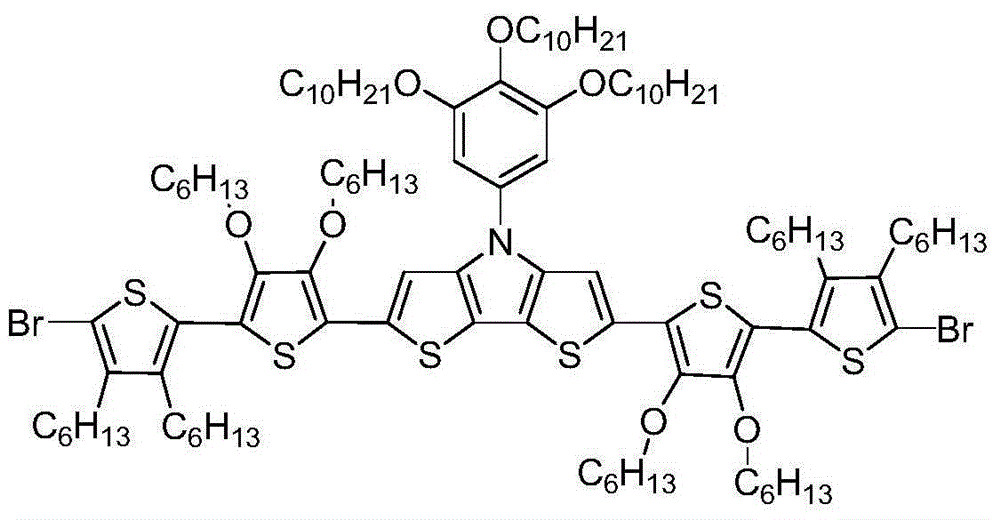
[0021] (1) Dibromo(bithiophene)-N-trialkoxyphenylbisthienopyrrole
[0022]
[0023] Add bis(bithiophene)-N-trialkoxyphenylbisthienopyrrole (2.0mmol), chloroform (60mL) and acetic acid (12mL) into a 250mL round bottom flask, add N-bromosuccinyl Amine (NBS, 2.1 mmol), the reaction mixture was stirred at room temperature for 3 h. Dichloromethane (100 mL) was then added to the reaction mixture, and the organic layer was dried over anhydrous magnesium sulfate after washing with water. The solvent was removed under reduced pressure, and the obtained light brown solid was the target product with a yield of 95%.
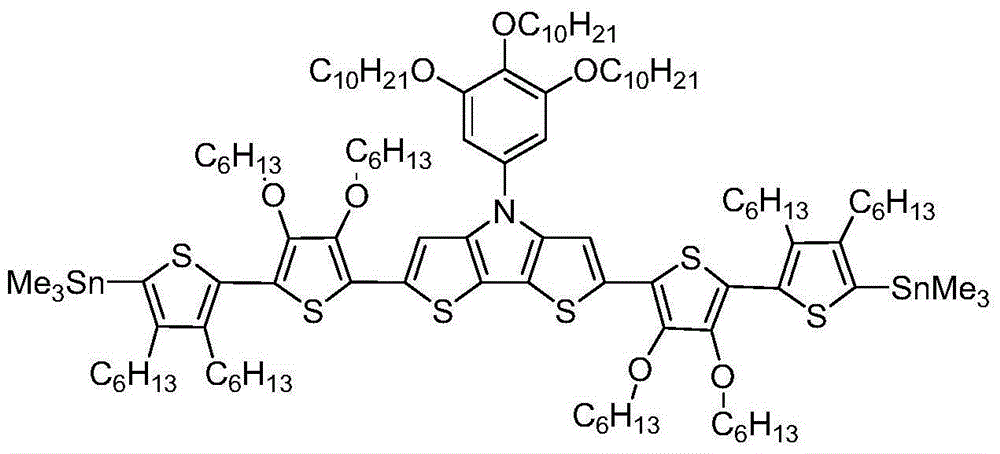
[0024] (2) Bis(2-trimethyltinthiophene)-N-trialkoxyphenylbisthienopyrrole
[0025]
[0026] Dibromo(bithiophene)-N-trialkoxyphenylbisthienopyrrole (1.22mmol) was dissolved in tetrahydrofuran (150mL), the reaction system was cooled to -78°C, and n-BuLi (2.68mmol ), stirred for 1 h, warmed to room temperature and then stirred for 2 h, cooled to -78 ° C again, and adde...
Embodiment 2
[0041] (1) Dibromo(bithiophene)-N-trialkoxyphenylbisthienopyrrole
[0042]
[0043] Add bis(bithiophene)-N-trialkoxyphenylbisthienopyrrole (2.0mmol), chloroform (60mL) and acetic acid (16mL) in a 250mL round bottom flask, add N-bromosuccinyl Amine (NBS, 2.2 mmol), the reaction mixture was stirred at room temperature for 0.5 h. Dichloromethane (180 mL) was then added to the reaction mixture, and the organic layer was dried over anhydrous magnesium sulfate after washing with water. The solvent was removed under reduced pressure to obtain a solid which was the target product with a yield of 91%.
[0044] (2) Bis(2-trimethyltinthiophene)-N-trialkoxyphenylbisthienopyrrole
[0045]
[0046] Dibromo(bithiophene)-N-trialkoxyphenylbisthienopyrrole (1.22mmol) was dissolved in tetrahydrofuran (100mL), the reaction system was cooled to -78°C, and n-BuLi (2.65mmol ) in n-hexane, stirred for 0.5h, slowly warmed up to room temperature and then stirred for 2h, cooled to -78°C again, ...
Embodiment 3
[0052] (1) Dibromo(bithiophene)-N-trialkoxyphenylbisthienopyrrole
[0053]
[0054] Add bis(bithiophene)-N-trialkoxyphenylbisthienopyrrole (2.0mmol), chloroform (60mL) and acetic acid (10mL) into a 250mL round bottom flask, add N-bromosuccinyl Amine (NBS, 2.1 mmol), the reaction mixture was stirred at room temperature for 2 h. Dichloromethane (100 mL) was then added to the reaction mixture, and the organic layer was dried over anhydrous magnesium sulfate after washing with water. The solvent was removed under reduced pressure, and the obtained light brown solid was the target product with a yield of 90%.
[0055] (2) Bis(2-trimethyltinthiophene)-N-trialkoxyphenylbisthienopyrrole
[0056]
[0057] Dibromo(bithiophene)-N-trialkoxyphenylbisthienopyrrole (1.22mmol) was dissolved in tetrahydrofuran (150mL), the reaction system was cooled to -78°C, and n-BuLi (2.7mmol ), stirred for 1 h, warmed to room temperature and then stirred for 2 h, cooled to -78 ° C again, and added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com