Superfine powder of Fibrates lipid-lowering drug and preparation method therefor
A technology of ultra-fine powder and fibrate, which is applied in the field of medicine, can solve problems such as poor process stability, large production capacity, and difficulty in maintaining the state, and achieve a production process with good parallelism, high bioavailability, and mild process conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
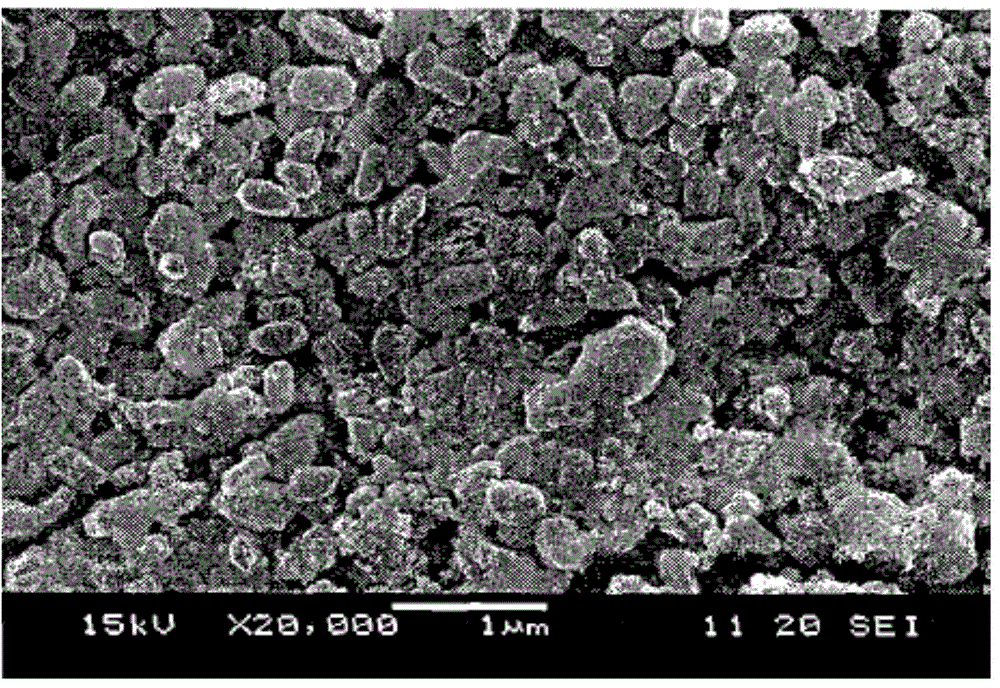
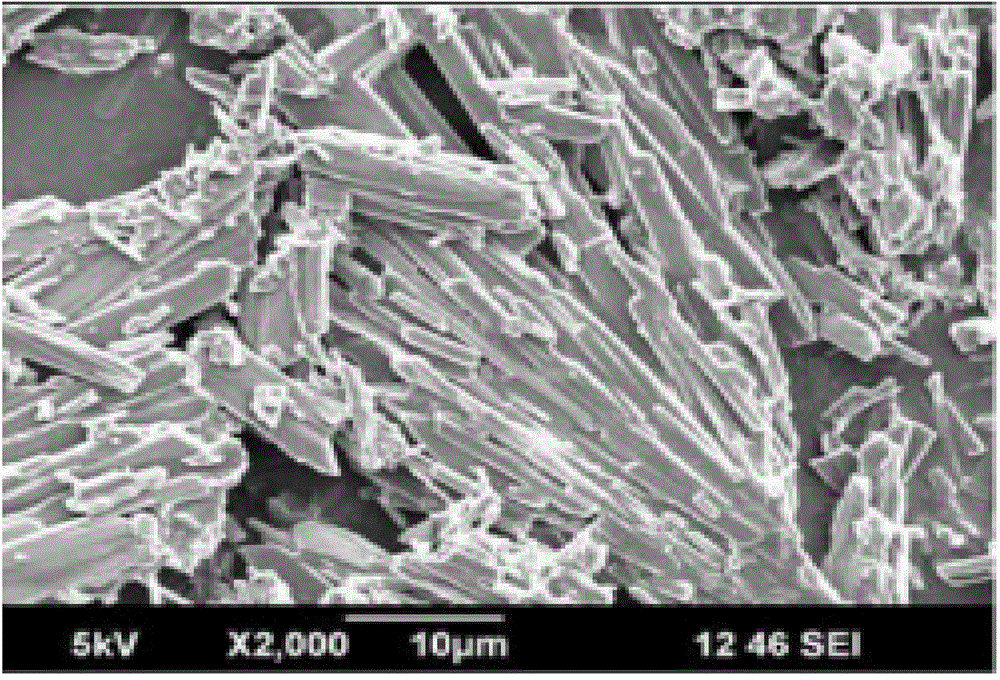
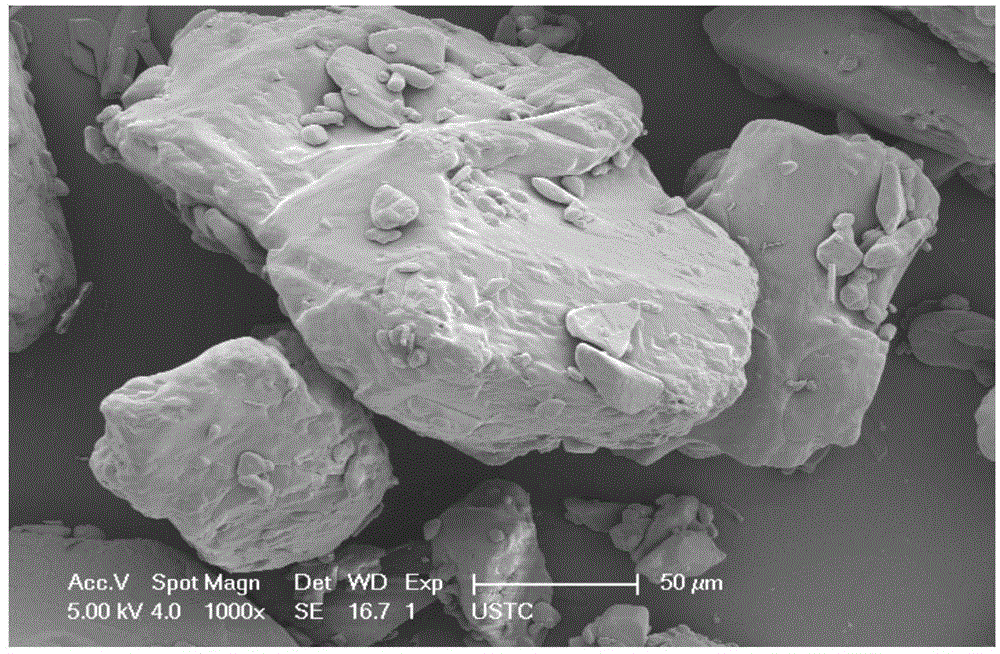
Image
Examples
Embodiment 1
[0048] 4g of gemfibrozil raw material was added to 50mL of methanol, heated to dissolve, cooled in an ice bath and simultaneously 20kHz, 200W ultrasonic to obtain white crystals; collected, washed and dried to obtain ultrafine powder of gemfibrozil.
Embodiment 2
[0050] Add 5 g of fenofibrate raw material, add 60 mL of absolute ethanol, heat to dissolve, cool down in an ice bath and simultaneously 30kHZ, 400W ultrasonic to obtain white crystals; collect, wash, and dry to obtain fenofibrate ultrafine powder.
Embodiment 3
[0052] 3g of bezafibrate raw material was added to 30mL of methanol, heated to dissolve, cooled in an ice bath and ultrasonicated at 20kHZ and 200W at the same time to obtain white crystals; collected, washed and dried to obtain bezafibrate superfine powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com