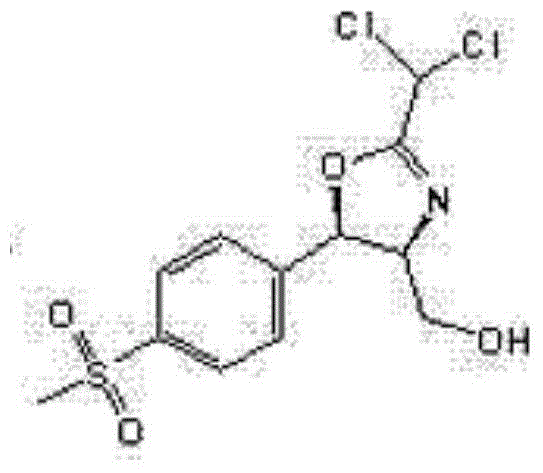
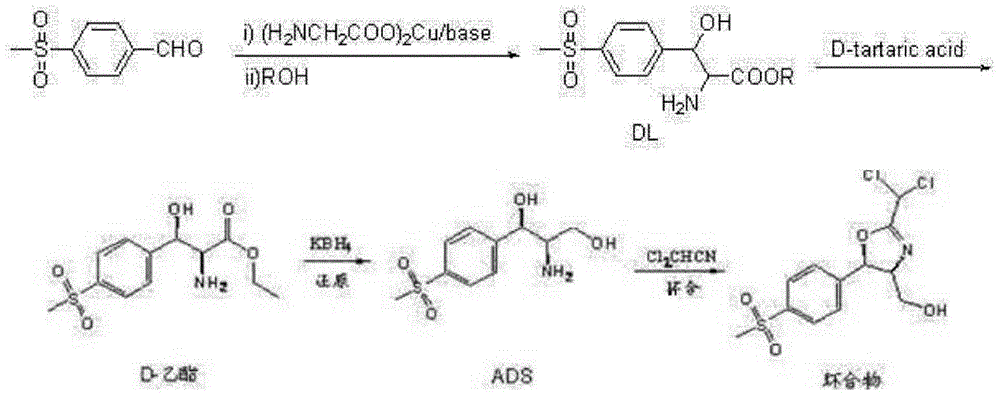
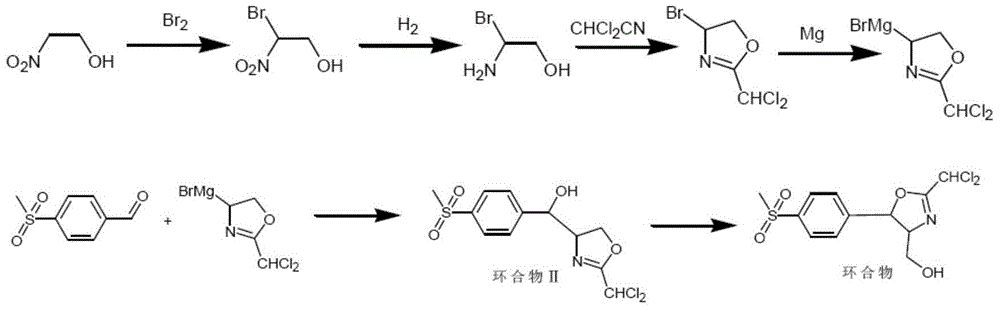
Synthetic method for (4R,5R)-2-bischloromethyl-4,5-dihydro-5-(4-methylsulfonyl)-4-oxazole methyl alcohol
A technology of methylsulfonyl phenyl and dichloromethyl is applied in the field of synthesizing florfenicol intermediates, and can solve the problems of difficult treatment of copper-containing wastewater, low total yield and high cost, and achieve high total yield, Improve efficiency and protect the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Take 91.1 g of 2-nitroethanol and add it to a four-necked flask with mechanical stirring, cool and control the temperature -10 to 0° C., slowly add 192.0 g of sodium hydroxide aqueous solution with a mass concentration of 25% under stirring, drop Continue to react for 20 minutes after the addition is complete, then add 191.8g of bromine dropwise, continue to complete the reaction after the dropwise addition, vacuum distillation and rectification separation to obtain 156.4g of 2-bromo-2-nitroethanol, the yield is 92.0%, the content 99.2%;
[0035] (2) Take 85.0 g of 2-bromo-2-nitroethanol in an autoclave, add 250.0 g of ethanol, drop in 5.0 g of palladium carbon, feed hydrogen to 0-0.1 MPa and supplement hydrogen during the reaction to keep the Pressure, react at 20-30°C until the pressure remains constant, filter the reaction solution to recover the catalyst, and rectify the filtrate to obtain 66.6g of 2-bromo-2-aminoethanol, with a yield of 95.1% and a content of 9...
Embodiment 2
[0040] (1) Get 91.1 g of 2-nitroethanol and add it to a four-necked flask with mechanical stirring, cool and control the temperature -10 to 0° C., slowly add 168.0 g of aqueous sodium hydroxide solution with a mass concentration of 25% under stirring, dropwise Continue to react for 20 minutes after the addition is complete, then add 167.8g of bromine dropwise, continue to complete the reaction after the dropwise addition, vacuum distillation and rectification separation to obtain 168.3g of 2-bromo-2-nitroethanol, the yield is 99.0%, the content 99.5%;
[0041] (2) Take 85.0 g of 2-bromo-2-nitroethanol in an autoclave, add 250.0 g of ethanol, drop in 5.0 g of palladium carbon, feed hydrogen to 0.1-0.2 MPa and supplement hydrogen during the reaction to keep the Pressure, react at 20-30°C until the pressure remains constant, filter the reaction solution to recover the catalyst, and rectify the filtrate to obtain 69.5g of 2-bromo-2-aminoethanol, with a yield of 99.3% and a content...
Embodiment 3
[0046] (1) Get 91.1 g of 2-nitroethanol and add it to a four-necked flask with mechanical stirring, cool and control the temperature -10 to 0° C., slowly add 144.0 g of aqueous sodium hydroxide solution with a mass concentration of 25% under stirring, drop Continue to react for 20 minutes after the addition, then add 143.8g of bromine dropwise, continue to complete the reaction after the dropwise addition, vacuum distillation and rectification separation to obtain 152.1g of 2-bromo-2-nitroethanol, the yield is 89.5%, the content 99.4%;
[0047] (2) Take 85.0 g of 2-bromo-2-nitroethanol in an autoclave, add 250.0 g of ethanol, drop in 5.0 g of palladium carbon, feed hydrogen to 0.1-0.2 MPa and supplement hydrogen during the reaction to keep the Pressure, react at 0-10°C until the pressure remains constant, filter the reaction solution to recover the catalyst, and rectify the filtrate to obtain 65.7g of 2-bromo-2-aminoethanol, with a yield of 93.8% and a content of 99.5%;
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com