Dehydroabietic acid benzimidazole Schiff base heterocyclic derivatives with anti-tumor activity and preparation method therefor and application thereof
A technology of dehydroabietic acid and benzimidazole, applied in the field of dehydroabietic acid benzimidazole Schiff base heterocyclic derivatives and its preparation, can solve the problems of killing cells, infection, bleeding, etc., and achieve novel structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
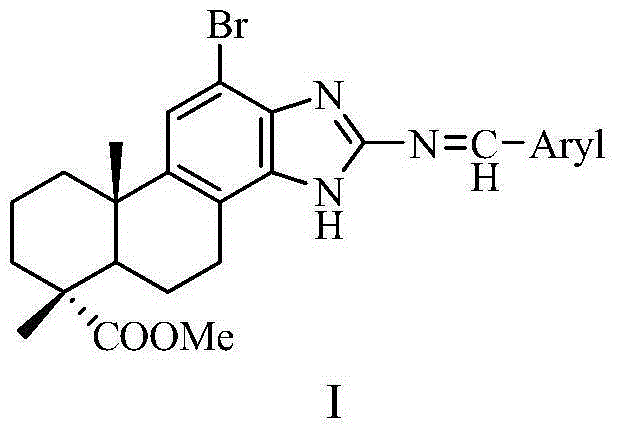
[0031] The preparation method of the dehydroabietic acid benzimidazole Schiff base heterocyclic derivatives having the structure shown in general formula I of the present invention comprises the following steps:
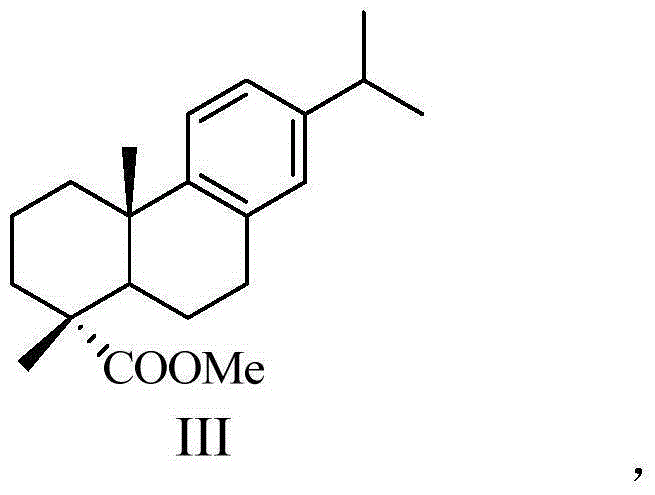
[0032] (1) dehydroabietic acid obtains dehydroabietic acid methyl ester through acyl chloride, methyl esterification reaction, has the structure shown in general formula III:
[0033]
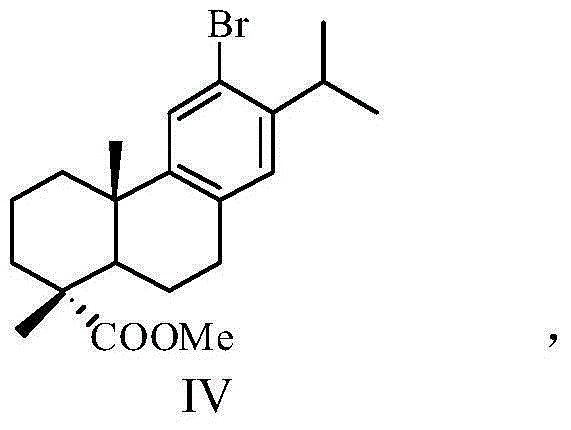
[0034] (2) Methyl dehydroabietate is brominated by NBS to obtain methyl dehydroabietate 12-bromo, which has a structure shown in general formula IV:
[0035]
[0036] (3) 12-bromodehydroabietic acid methyl ester is double-nitrated with fuming nitric acid to obtain 12-bromo-13,14-dinitrodeisopropyl dehydromethyl ester, which has the structure shown in general formula V:
[0037]
[0038] (4) 12-bromo-13,14-dinitro deisopropyl dehydroabietic acid methyl ester is reduced by Fe / HCl to obtain 12-bromo-13,14-diamino deisopropyl dehydroabietic acid methyl ester, which has Structure...
Embodiment 1
[0048] Synthesis of Methyl Dehydroabietate (III)
[0049] In a 500mL three-necked round-bottom flask, dissolve 30g (0.1mol) of dehydroabietic acid in 100mL of benzene, slowly add 10.9mL of thionyl chloride (0.15mol) and heat to reflux for 3h. After the reaction, remove the reaction solution under reduced pressure Benzene and excess thionyl chloride to obtain dehydroabietic acid chloride as yellow oil. Add 60mL of methanol into the bottle, heat to reflux for 3h, after the reaction, remove the solvent under reduced pressure, and recrystallize from ethanol to obtain white needle-like crystals which are methyl dehydroabietate (30.63g, 97.6%), m.p.62.3~63.9℃ .
Embodiment 2
[0051] Synthesis of 12-bromodehydroabietic acid methyl ester (IV)
[0052] Dissolve 15g of methyl dehydroabietate in 100mL of dry acetonitrile, then add 12g of NBS to the mixed solution, and react in the dark for 24 hours at room temperature, evaporate the solvent acetonitrile under reduced pressure, and add 100mL of carbon tetrachloride while hot , after cooling, filter out the insolubles in the solution, evaporate the solvent carbon tetrachloride under reduced pressure, dissolve it with anhydrous methanol, and recrystallize to obtain 10.5 g of white needle-like crystals, which is 12-bromodehydroabietic acid methyl Ester, yield 67%, m.p.133.5~135.7℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com