Preparation method of epirubicin and its intermediate
A technology of epirubicin and trifluoroacetyl epirubicin, applied in the field of organic compound synthesis, can solve the problems of bromination reaction pollution, large environmental damage, numerous steps and the like, achieve fewer reaction steps and avoid serious pollution , the effect of reducing economic and environmental costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
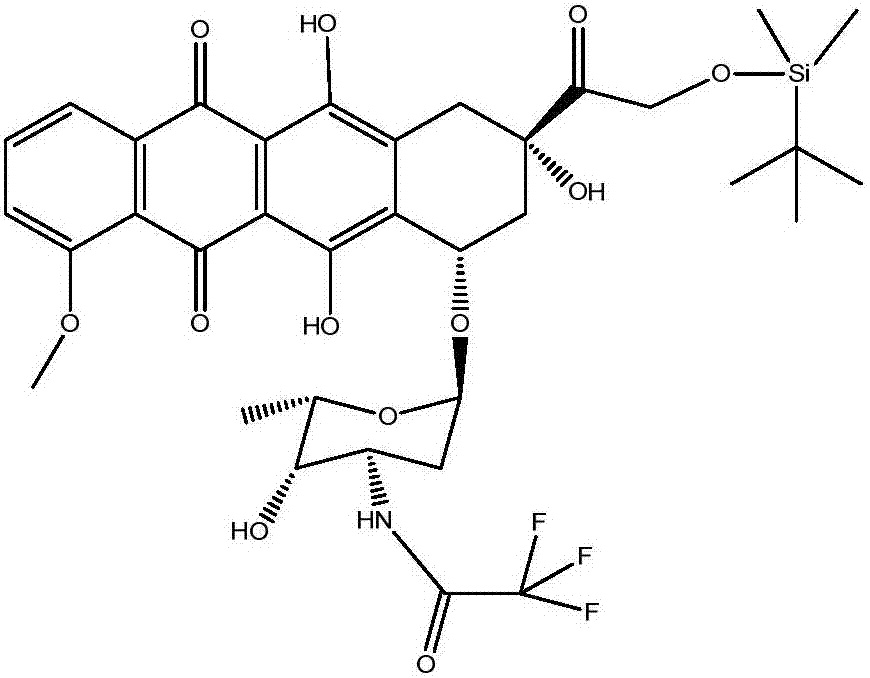
[0062] Example 1: Synthesis of N-trifluoroacetyl adriamycin
[0063] (a) Suspend 5 grams of doxorubicin in 200ml DCM and cool to 0°C, stir and slowly add dropwise a mixture of 8ml DCM and 15ml trifluoroacetic anhydride within 30 minutes, and stir and react for 30 minutes after completion;
[0064] (b) Add methanol to the obtained mixed solution at 0°C, stir for 30 minutes, and warm to room temperature;
[0065] (c) Add 200ml of saturated sodium bicarbonate to the mixed solution, and stir at room temperature for 10 hours to carry out the hydrolysis reaction;
[0066] (d) After completion of the hydrolysis (monitored by HPLC), separate the organic layer, dehydrate the organic phase and concentrate it to dryness under reduced pressure to obtain an oil, weighing 6 g, in which N-trifluoroacetyl adriamycin measured by HPLC The chromatographic purity of is 93%, all of which were directly used in the reaction of the following examples (no further purification is required, the same in the foll...
Embodiment 2
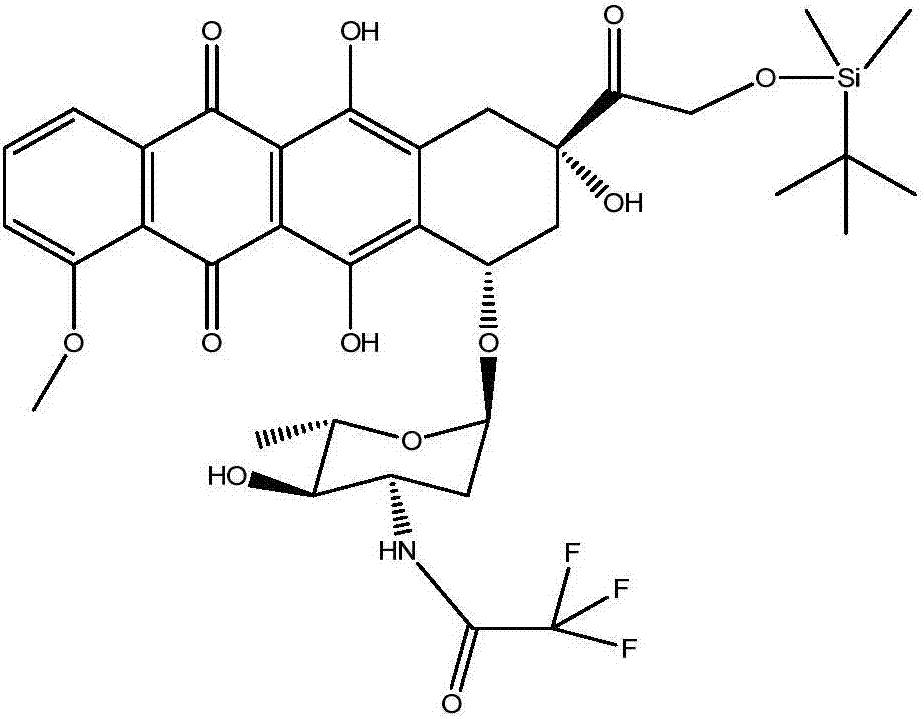
[0067] Example 2: Preparation of N-trifluoroacetyl-14-O-tert-butyldimethylsilyl adriamycin (compound 1)
[0068] (a) Add 50ml of DMF to dissolve the concentrate obtained in Example 1, then add 5g of dried molecular sieve and 1g of imidazole, stir and dissolve;
[0069] (b) Add a mixed solution of 1.5g tert-butyldimethylchlorosilane and 10ml DMF to the mixture obtained above at 25°C, and react for 6 hours;
[0070] (c) Add 200 ml of DCM and 100 ml of HCl aqueous solution with a pH of 2.0 to the reaction solution, and stir and separate. The organic phase was washed with 100 ml of water again, the organic layer was separated, and after dehydration, the organic phase was concentrated under reduced pressure and concentrated to dryness to obtain 6.0 g of solid. The N-trifluoroacetyl-14-O-tert-butyldimethyl was measured by HPLC. The chromatographic purity of silyl adriamycin is 91%.
[0071] All were directly used in the reaction of Example 3 below.
[0072] 1 H-NMR (400MHZ, DMSO):
[0073] 0...
Embodiment 3
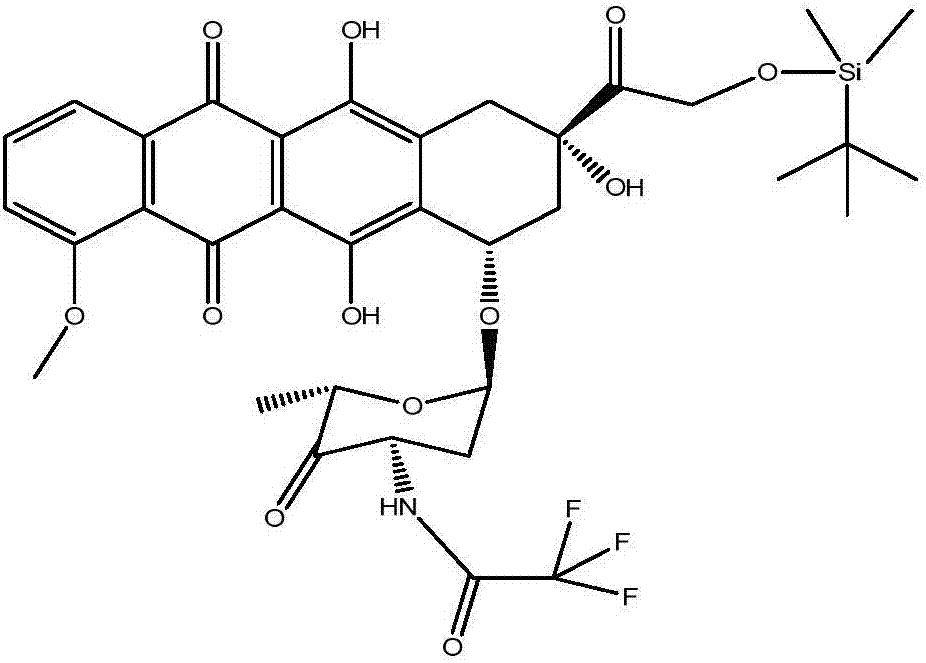
[0074] Example 3: Preparation of 4'-keto-N-trifluoroacetyl-14-O-tert-butyldimethylsilyl adriamycin (compound 2)
[0075] (a) Mix 100ml of DCM and 5ml of DMSO under stirring, and cool to -60°C, then add a mixture of 5ml of trifluoroacetic anhydride and 10ml of DCM, and stir for 15min;
[0076] (b) Mix 6.0 g of N-trifluoroacetyl-14-O-tert-butyldimethylsilyl adriamycin obtained in Example 2 with 50 ml of DCM, and then add dropwise to the mixed solution obtained in step (a) , The dripping time is 30min, the temperature during the dripping process is controlled to -65℃~-60℃, after the dripping, continue to react at -65℃~-60℃ for 45 minutes;
[0077] (c) Add 5ml of DBN solution and continue to react at -65℃~-60℃ for 10 minutes;
[0078] (d) Add 3ml of glacial acetic acid to neutralize the reaction solution, then add 100ml of water, stir and separate the liquids, and then wash with 100ml of water once to separate the organic phase and dehydrate;
[0079] (e) After concentration under reduced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com