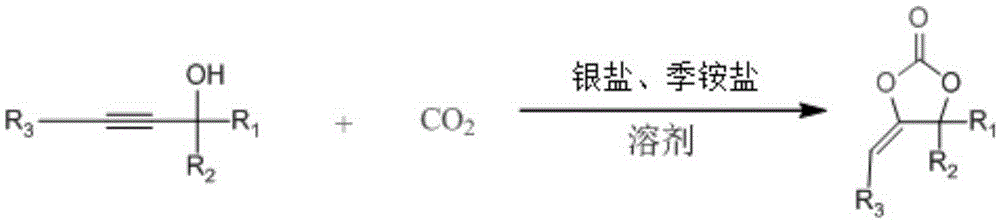
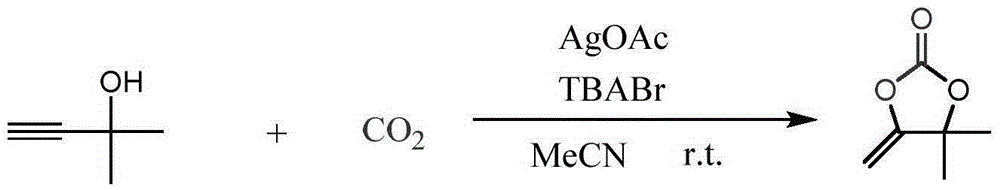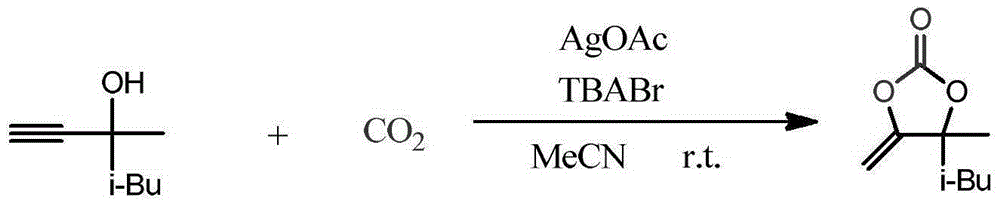Method for synthesizing cyclic carbonate through CO<2> and propargyl alcohol
A technology of cyclic carbonate and propargyl alcohol, applied in the field of organic chemical synthesis, can solve the problems of complex preparation process, high production cost and equipment requirements, large catalyst consumption, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Place 2mmol of 2-methyl-3-butyn-2-ol (2-methylbut-3-yn-2-ol, 1a) with 0.7mmol of AgOAc and 2mmol of TBABr in a round-bottomed flask containing 20mL of acetonitrile solution at room temperature and pressure CO 2 Carry out the synthetic reaction of following structural formula:
[0017]
[0018] Stir until the end of the reaction (TLC plate to determine the end point of the reaction), rotary evaporate the solvent, add 5mL of water, extract 3 times with 15mL of anhydrous ether, dry for 3h and then concentrate the solvent to obtain the target product as cyclic carbonate— — 4,4-dimethyl-5-methenyl-1,3-dioxan-2-one (4,4-dimethyl-5-methylene-1,3-dioxolan-2-one, 2a), Its yield is 98%.
Embodiment 2
[0020] Place 2 mmol 3,5-dimethylhex-1-yn-3-ol (3,5-dimethylhex-1-yn-3-ol, 1b) with 0.7 mmol AgOAc and 2 mmol TBABr in a round bottom flask containing 20 mL of acetonitrile solution , CO was introduced at room temperature and pressure 2 Carry out the synthetic reaction of following structural formula:
[0021]
[0022] Stir until the end of the reaction (TLC spotting to determine the end point of the reaction), add 5 mL of water after rotary evaporation to remove the solvent, extract 3 times with 15 mL of anhydrous ether, dry for 3 hours and then concentrate the solvent to obtain the target product cyclic carbonate—— 4-Methyl-4-isobutyl-5-methenyl-1,3-dioxan-2-one (4-isobutyl-4-methyl-5-methylene-1,3-dioxolan-2-one , 2b) with a yield of 94%.
Embodiment 3
[0024] Put 2mmol acetylenic alcohol 3-ethyl-1-phenylhept-1-yn-3-ol (3-ethyl-1-phenylhept-1-yn-3-ol, 1c) with 0.7mmol AgOAc and 2mmolTBABr in 20mL In a round-bottomed flask of acetonitrile solution, CO was introduced at room temperature and pressure. 2 Carry out the synthetic reaction of following structural formula:
[0025]
[0026] Stir until the end of the reaction (TLC spotting to determine the end point of the reaction), add 5 mL of water after rotary evaporation to remove the solvent, extract 3 times with 15 mL of anhydrous ether, dry for 3 hours and then concentrate the solvent to obtain the target product cyclic carbonate—— 4-Ethyl-4-butyl-5-benzylidene-1,3-dioxolan-2-one (5-benzylidene-4-butyl-4-ethyl-1,3-dioxolan-2-one , 2c) with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com