A kind of lzc696 intermediate and its synthetic method
A compound and reaction system technology, which is applied in the field of intermediates for the synthesis of NEP inhibitors, can solve the problems of sensitivity, high production costs, and unrecoverable palladium heavy metal catalysts, etc., and achieve the effect of low process costs and simple purification procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] This embodiment discloses a method for producing a compound of formula A
[0064]
[0065] Include the following steps:
[0066] a) Take the compound D-serine methyl ester hydrochloride (1000g, 6.45mol, 1eq) and dissolve it in 5L of dichloromethane, add triethylamine (1955g, 19.35mol, 3eq), keep the temperature at -5~5℃, divide Add triphenylchloromethane (2152g, 7.74mol, 1.2eq) in batches, stir at room temperature until the reaction is complete, add water to quench, separate the layers, wash the organic phase with hydrochloric acid, and use anhydrous sodium sulfate when TLC detection confirms that there is no triethylamine After drying and concentrating the solvent, a white solid was obtained which was N-trityl-D-serine methyl ester. Yield 92%. The nuclear magnetic and mass spectrum data of product in this step are as follows:
[0067] 1H NMR (300MHz, CDCl3) 7.34(s, 6H), 7.23(d, 6H), 7.21(m, 3H), 5.23(s, 1H), 4.23(m, 1H), 3.87(m, 1H), 3.72 (m,1H)3.62(m,3H),3.52(m...
Embodiment 2
[0081] The compound A obtained by the method in Example 1 is used to prepare compound B, specifically:
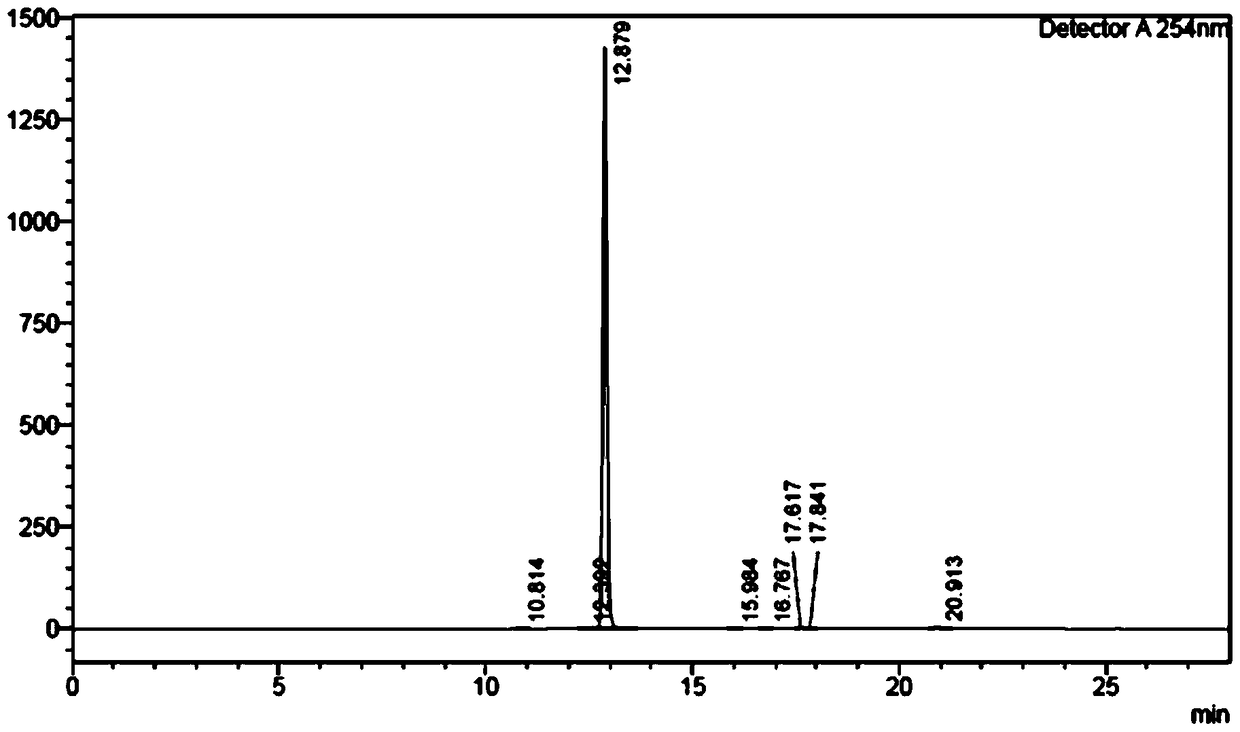
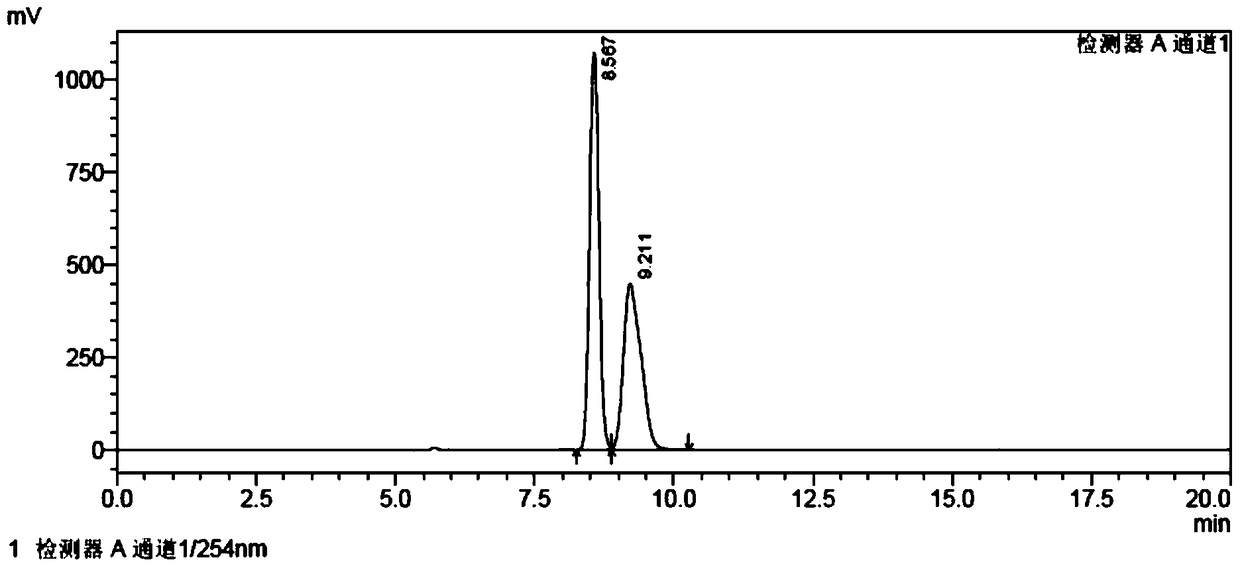
[0082] (R)-tert-butyl(1-((1,1'biphenyl)-4-yl)3-((tert-butyldimethylsilyl)oxy)propan-2-yl)carbamate (1000g, 2.26mol, 1eq) was dissolved in 6L of dichloromethane, cooled to -10°C and slowly added tetrabutylammonium fluoride (540g, 2.06mol, 0.9eq), after the reaction, sodium bicarbonate solution was added, and the liquid was separated , the organic phase was washed with salt water, separated, the organic phase was dried with anhydrous sodium sulfate, and the organic phase was concentrated to obtain a white powder which was LZC696 intermediate (R)-tert-butyl (1-([1,1'-biphenyl ]-4-yl)-3-hydroxypropan-2-yl)carbamate. Yield 90%, purity 99%, EE>99%. The nuclear magnetic data and mass spectrum data of product in this step are as follows:
[0083]1HNMR (300MHz, DMSO) 7.65(m,1H),7.63(m,1H),7.58(m,1H),7.56(m,1H),7.47(s,1H),7.45(s,1H),7.28( s,1H),7.43(s,1H),7.36(s,1H),7.34(s,1H),6....
Embodiment 3
[0089] This embodiment discloses a method for producing a compound of formula A, comprising the following steps:
[0090] a) Dissolve the compound D-serine methyl ester hydrochloride (10g, 64.5mmol, 1eq) in 100mL of dichloromethane, add triethylamine (19.6g, 193.5mmol, 3eq), keep the temperature at 45-50°C, divide Add triphenylchloromethane (21.5g, 77.4mmol, 1.2eq) in batches, stir at room temperature until the reaction is complete, add water to quench, separate the layers, wash the organic phase with hydrochloric acid, and use anhydrous sulfuric acid when it is confirmed by TLC that there is no triethylamine Drying over sodium and concentrating the solvent gave a white solid which was N-trityl-D-serine methyl ester. Yield 89%. The nuclear magnetic and mass spectrum data of product in this step are as follows:
[0091] 1H NMR (300MHz, CDCl3) 7.35(s, 6H), 7.23(d, 6H), 7.21(m, 3H), 5.24(s, 1H), 4.23(m, 1H), 3.87(m, 1H), 3.72 (m,1H)3.62(m,3H),3.52(m,1H)MS: m / z=362.2(M+H)+;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com