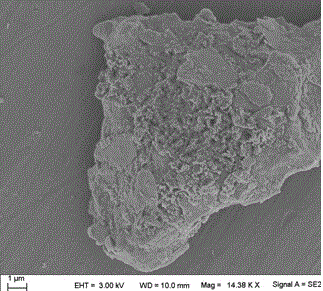
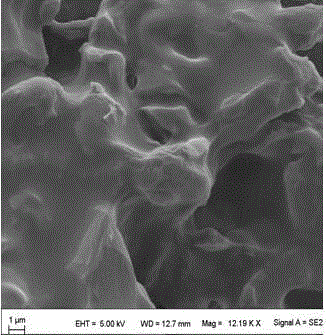
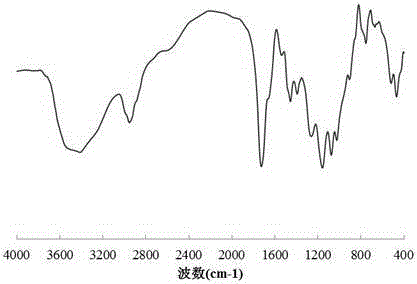
Preparation method of loess and itaconic acid copolymer adsorbent
A technology of itaconic acid copolymer and adsorbent, applied in chemical instruments and methods, adsorption of water/sewage treatment, water pollutants, etc., to achieve good biocompatibility, enhanced chelation ability and ion exchange ability, and good adsorption effect of ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of loess itaconic acid copolymer compound: In a 100mL three-necked flask, add 4g loess (LC) and 20mL distilled water, stir and disperse evenly; dissolve 4g itaconic acid (IA) in 20mL distilled water and add to the three-necked flask , stir and disperse evenly; then add 6g of methacrylic acid β- Hydroxyethyl ester (HEMA), 4g N - Vinylpyrrolidone (NVP), 0.6g N,N ’-Methylenebisacrylamide (MBA), stir and disperse evenly; then N 2 Protect, heat up to 50°C; add 0.5g potassium persulfate, stir and react for 8 hours, wash and dry the complex to obtain loess itaconic acid copolymer complex.
[0030] The effect of the loess itaconic acid copolymer on Cu in water 2+ The removal rate reaches 96.5%, and the adsorption capacity is 176.8mg / g; the decolorization rate for malachite green reaches 96.1%, and the adsorption capacity is 96.1mg / g.
Embodiment 2
[0032] Preparation of loess itaconic acid copolymer compound: In a 100mL three-necked flask, add 4g loess (LC) and 20mL distilled water, stir and disperse evenly; dissolve 2g itaconic acid (IA) in 20mL distilled water and add to the three-necked flask , stir and disperse evenly; then add 4g of methacrylic acidβ- Hydroxyethyl ester (HEMA), 4g N - Vinylpyrrolidone (NVP), 0.8g N,N ’-Methylenebisacrylamide (MBA), stir and disperse evenly; then N 2 Protect, heat up to 90°C, add 0.7g of ammonium persulfate, stir and react for 5 hours, wash and dry the complex to obtain loess itaconic acid copolymer complex.
[0033] The effect of the loess itaconic acid copolymer on Cu in water 2+ The removal rate reached 95.2%, and the adsorption capacity was 168.9mg / g. The decolorization rate of malachite green reaches 93.4%, and the adsorption capacity is 93.4mg / g.
Embodiment 3
[0035] Preparation of loess itaconic acid copolymer compound: In a 100mL three-necked flask, add 4g loess (LC) and 20mL distilled water, stir and disperse evenly; dissolve 4g itaconic acid (IA) in 20mL distilled water and add to the three-necked flask , stir and disperse evenly; then add 4g of methacrylic acid β- Hydroxyethyl ester (HEMA), 4g N - Vinylpyrrolidone (NVP), 0.8g N,N ’-Methylenebisacrylamide (MBA), stir and disperse evenly; then N 2 Protect, heat up to 70°C; add 0.7g of ammonium persulfate, stir and react for 6 hours to obtain a copolymer, and then wash and dry to obtain a loess itaconic acid copolymer compound.
[0036] The effect of the loess itaconic acid copolymer on Cu in water 2+ The removal rate reached 98.2%, and the adsorption capacity was 178.8mg / g; the malachite green decolorization rate reached 97.0%, and the adsorption capacity was 97.0mg / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com