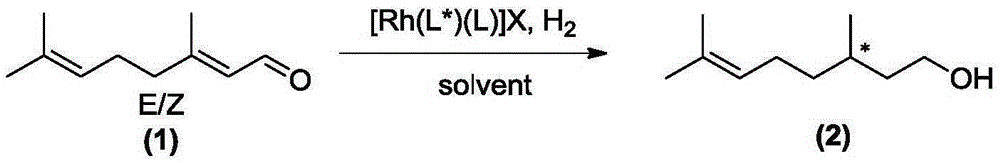Method for preparing chiral citronellol through asymmetric catalytic hydrogenation of citral
A catalytic hydrogenation, asymmetric technology, applied in the direction of organic chemical methods, chemical instruments and methods, preparation of hydroxyl compounds, etc., to achieve the effects of cost reduction, easy industrial production, and control of chirality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of Chiral Citronellol
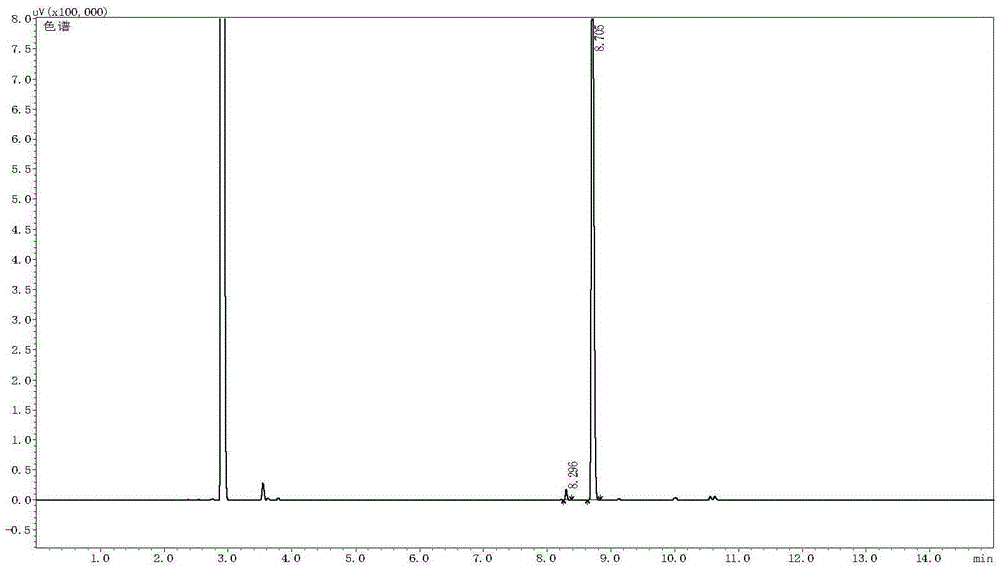
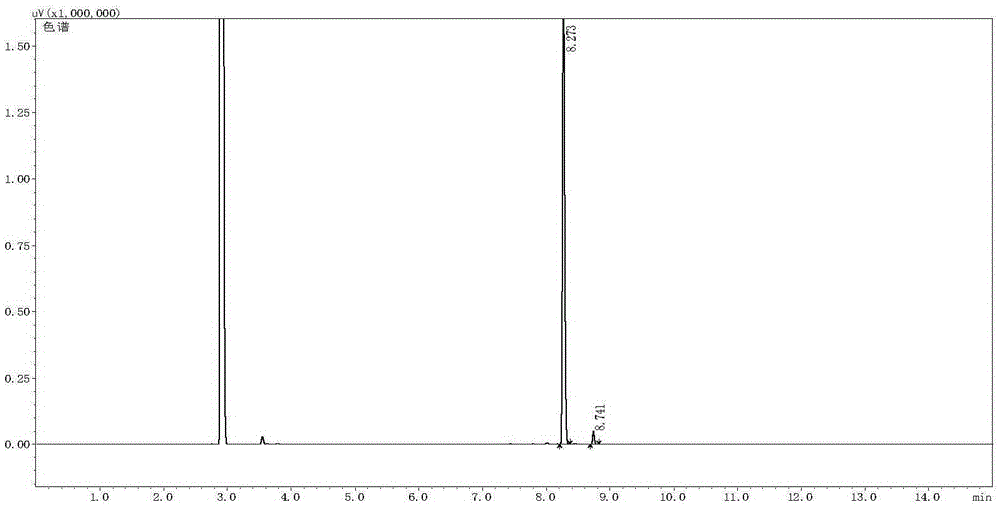
[0046] In a 10 mL reaction tube, add the phosphine ligand R-L1a (3.5 mg, 0.005 mmol) and bis(1,5-cyclooctadiene) rhodium tetrafluoroborate [Rh(COD) 2 ]BF 4 (0.005 mmol), the system was passed through a vacuum line, replaced with nitrogen three times, freshly distilled degassed toluene (2 mL) was added, the solution was stirred at room temperature for 1 hour, the solvent was removed under reduced pressure to obtain a brown solid, and after vacuum extraction for 2 hours, 2 mL of methanol solvent was added, and the catalyst solution was added with E-form citral (0.50 mmol, E / Z=99 / 1, chiral rhodium complex [Rh(R-L1a)(COD)]BF) 4 The molar ratio of citral to citral was 1 / 100), put it into an autoclave, and after 6 hydrogen replacements, the initial hydrogen pressure (gauge pressure) was 45 bar, and the reaction was stirred at 60° C. for 5 hours. Cool, release the gas, open the autoclave, take out the vial, drain the solvent, check the con...
Embodiment 2
[0048] Preparation of Chiral Citronellol
[0049] In a 10 mL reaction tube, add the phosphine ligand S-L1b (4.1 mg, 0.005 mmol) and bis(1,5-cyclooctadiene) rhodium tetrafluoroborate [Rh(COD) 2 ]BF 4 (2.1 mg, 0.005 mmol), the system was passed through a vacuum line, replaced with nitrogen three times, freshly distilled degassed toluene (2 mL) was added, the solution was stirred at room temperature for 1 hour, and the solvent was removed under reduced pressure to obtain a brown solid, which was vacuumed for 2 After 1 hour, 2 mL of methanol solvent was added, and the catalyst solution was added with E-form citral (76.1 mg, 0.50 mmol, E / Z=99 / 1, chiral rhodium complex [Rh(S-L1b)(COD)] BF 4 The molar ratio of citral to citral was 1 / 100), put it into an autoclave, and after 6 hydrogen replacements, the initial hydrogen pressure was 10 bar, and the reaction was stirred at 0° C. for 36 hours. Cool, carefully release the gas, open the autoclave, take out the vial, drain the solvent, ...
Embodiment 3
[0051] Preparation of Chiral Citronellol
[0052] In a 10 mL reaction tube, add the phosphine ligand R-L1c (4.8 mg, 0.005 mmol) and bis(2,5-norbornadiene) rhodium tetrafluoroborate [Rh(NBD) 2 ]BF 4 (1.9 mg, 0.005 mmol), the system was passed through a vacuum line, replaced with nitrogen three times, freshly distilled degassed toluene (2 mL) was added, the solution was stirred at room temperature for 1 hour, and the solvent was removed under reduced pressure to obtain a brown solid, which was vacuumed for 2 After 1 hour, 2 mL of methanol solvent was added, and this solution was added with Z-form citral (76.1 mg, 0.5 mmol, E / Z=1 / 99, chiral rhodium complex [Rh(R-L1c)(NBD)]BF 4 The molar ratio of citral to citral was 1 / 100), put it into an autoclave, and after 6 hydrogen replacements, the initial hydrogen pressure was 40 bar, and the reaction was stirred at 45° C. for 8 hours. Cool, carefully release the gas, open the autoclave, take out the vial, drain the solvent, check the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com