Biological coating based on cyclomatrix-type polyphosphazene and preparing method thereof
A bio-coating and polyphosphazene technology, applied in coatings, anti-corrosion coatings, etc., can solve the problems of functional monomer limitations, achieve high reaction efficiency, simple preparation conditions, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The ring-crosslinked polyphosphazene is applied to the surface modification of the topological honeycomb polystyrene material. This example is implemented under the conditions of implementation and technical requirements.
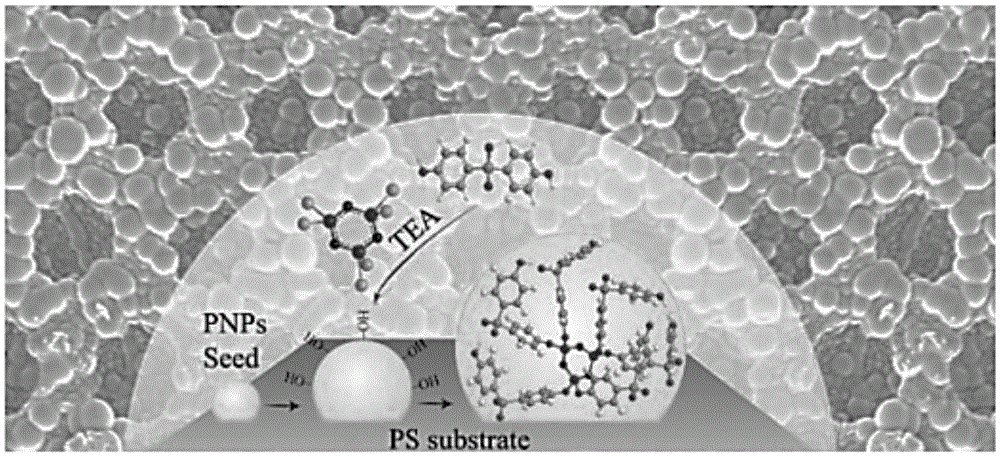
[0033] 1. If figure 1 and Figure 5 Shown is the process of preparing polyphosphazene coating on topological honeycomb polystyrene surface:
[0034] 1) Dissolve 1 g of polystyrene in 20 mL of toluene, and prepare a honeycomb porous polystyrene membrane on a clean glass substrate according to the breathing pattern method provided in the literature [EscaleP. etal, European Polymer Journal 2012, 6, 1001] for future use.
[0035] 2) 4,4-dihydroxydiphenyl sulfone is used as a multifunctional monomer.
[0036] 3) Weigh 20mg of hexachlorocyclotriphosphazene and 44mg of 4,4‐dihydroxydiphenylsulfone, the molar ratio is (1:3), dissolve in 8mL of absolute ethanol, and disperse evenly by ultrasonic.
[0037] 4) Place the pre-prepared topological honeycomb pol...
Embodiment 2
[0051] The ring-crosslinked polyphosphazene is applied to the surface modification of polystyrene materials. This example is implemented under the conditions of implementation and technical requirements.
[0052] 1. The process of preparing polyphosphazene coating on polystyrene surface:
[0053] 1) Dissolve 1 g of polystyrene in 20 mL of toluene, and drop the obtained solution on a clean glass substrate to prepare a polystyrene film for future use.
[0054] 2) 4,4-dihydroxydiphenyl sulfone is used as a multifunctional monomer.
[0055] 3) Weigh 20 mg of hexachlorocyclotriphosphazene and 43 mg of 4,4‐dihydroxydiphenyl sulfone in a molar ratio of (1:3), dissolve them in 8 mL of absolute ethanol, and disperse evenly by ultrasonication.
[0056] 4) Place the pre-prepared polystyrene in a clean glass petri dish, add the solution in step 3) into the petri dish, and ensure that the solution completely submerges the substrate.
[0057] 5) Add 200 μL of anhydrous triethylamine as an...
Embodiment 3
[0068] The ring-crosslinked polyphosphazene is applied to the surface modification of polyethylene materials. This example is implemented under the conditions of implementation and technical requirements.
[0069] 1. The process of preparing polyphosphazene coating on polyethylene surface:
[0070] 1) Buy a polyethylene board and keep it as a spare.
[0071] 2) 4,4-dihydroxydiphenyl sulfone is used as a multifunctional monomer.
[0072] 3) Weigh 20 mg of hexachlorocyclotriphosphazene and 43 mg of 4,4‐dihydroxydiphenyl sulfone in a molar ratio of (1:3), dissolve them in 8 mL of absolute ethanol, and disperse evenly by ultrasonication.
[0073] 4) Place the pre-prepared polyethylene in a clean glass petri dish, add the solution in step 3) into the petri dish, and ensure that the solution completely submerges the substrate.
[0074] 5) Add 200 μL of anhydrous triethylamine as an acid-binding agent, and cover the lid of the culture dish.
[0075] 6) Stir at room temperature for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com