Enrofloxacin injection and preparation method thereof
A technology of enrofloxacin and injection, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of low formulation cost and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention provides the preparation method of above-mentioned enrofloxacin injection, comprises the following steps:
[0050] (1) After heating part of the water for injection, mix it with enrofloxacin, nicotinamide, an organic solvent, disodium edetate and an antioxidant, and dissolve to obtain a mixed material;
[0051] (2) Adjusting the pH value of the mixed material in step (1) with a pH regulator and mixing it with the rest of the water for injection to obtain enrofloxacin injection.
[0052] In the present invention, part of the water for injection is heated and mixed with enrofloxacin, nicotinamide, organic solvent, disodium ethylenediaminetetraacetic acid and antioxidant to obtain a mixed material after dissolution. In the present invention, it is preferred to heat part of the water for injection to 75-85°C, then mix it with enrofloxacin, nicotinamide, organic solvent, disodium edetate and antioxidant, and obtain the mixed material after dissolving. ...
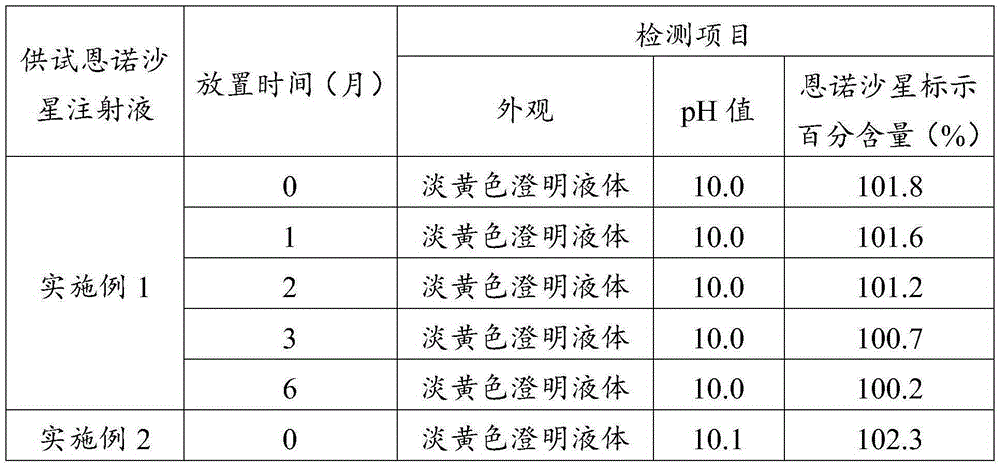
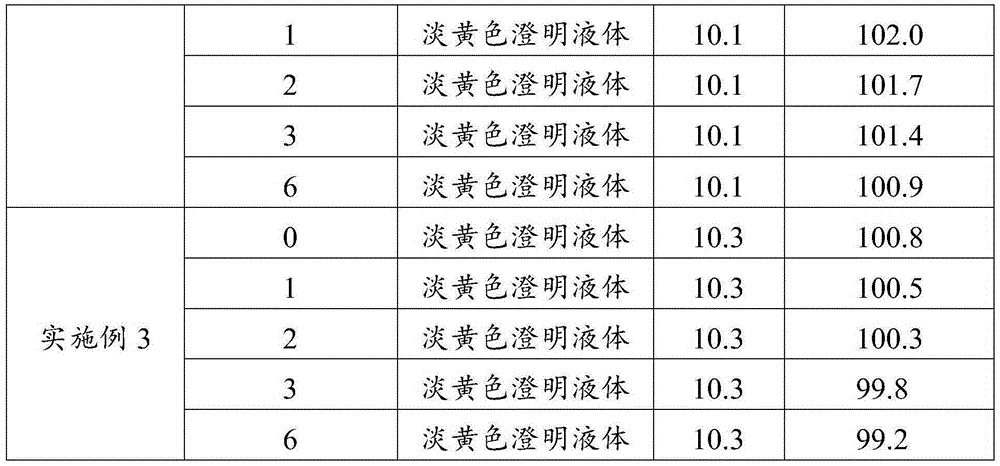
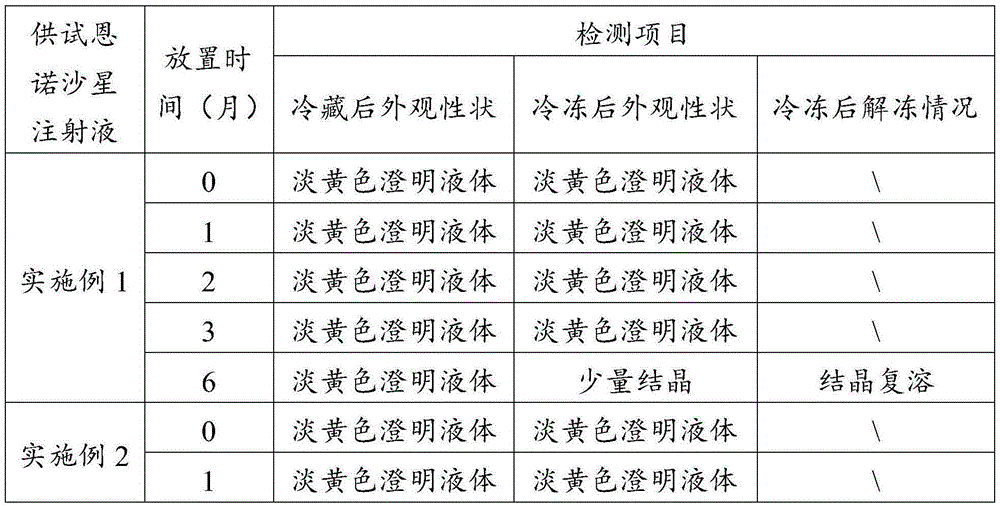
Embodiment 1
[0061] (1) Take 60 parts of water for injection and heat it to 75°C, mix it with 10 parts of propylene glycol, 2.5 parts of enrofloxacin, 2 parts of nicotinamide, 0.01 part of edetate disodium and 0.1 part of sodium thiosulfate, and stir to make dissolved to obtain a mixture.
[0062] (2) Use ethanolamine to adjust the pH value of the mixed material to 9.5, add the remaining water for injection to make up to the full amount, and obtain enrofloxacin injection.
[0063] (3) Test the obtained enrofloxacin injection, filter the qualified enrofloxacin injection with a 0.45 μm microporous membrane, fill and seal it in an ampoule, and then extinguish it under circulating steam at 110°C. Bacteria for 40 minutes, cooled to room temperature, packaged, and stored for inspection.
Embodiment 2
[0065] (1) Propylene glycol and ethanol are prepared into a mixed solvent according to the proportion range; take 50 parts of water for injection and heat it to 80°C, and mix it with 20 parts of mixed solvent, 5 parts of enrofloxacin, 4 parts of nicotinamide, and 0.03 parts of ethylenediaminetetraacetic acid Disodium and 0.3 parts of sodium formaldehyde sulfoxylate are mixed and stirred to dissolve to obtain a mixed material; wherein the volume ratio of propylene glycol and ethanol in the mixed solvent is 1.5:1.
[0066] (2) Use 10% sodium hydroxide solution to adjust the pH value of the mixed material to 10, add the remaining water for injection to make up to the full amount, and obtain enrofloxacin injection.
[0067] (3) Test the obtained enrofloxacin injection, filter the qualified enrofloxacin injection with a 0.45 μm microporous membrane, fill and seal it in an ampoule, and then extinguish it under steam at 115°C. Bacteria for 35 minutes, cooled to room temperature, pack...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com