Cleaning production method for iminodiacetonitrile
A technology of iminodiacetonitrile and hydroxyacetonitrile, which is applied in the chemical industry, can solve problems affecting product quality, complicate production, limit the scope of use, etc., and achieve the effect of improving product appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
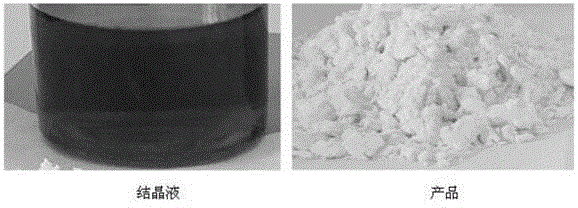
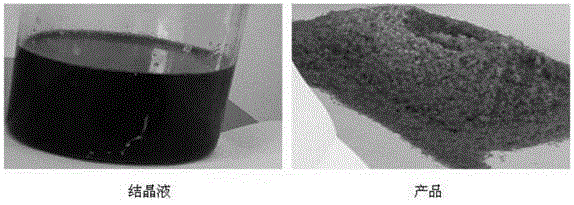
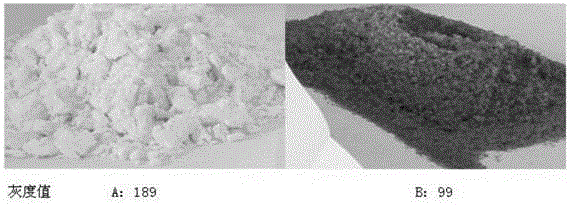
[0079] Take 1000 g of iminodiacetonitrile reaction solution produced by Xinjiang Ziguang Yongli Chemical Industry Co., Ltd., which is brown-black, with an iminodiacetonitrile content of 25%, quickly cool down to 45°C, feed sulfur dioxide gas until the reaction solution pH=3.0, and cool down to 10-15°C. ℃, stirred and crystallized for 6.0 h, filtered, washed the solid with a small amount of water, and dried to obtain 238.6 g of white iminodiacetonitrile product, the content of iminodiacetonitrile was 98.3%, and the impurity content of compound I was 0.38%.
Embodiment 2
[0085] Take 1000 g of iminodiacetonitrile reaction solution produced by Xinjiang Ziguang Yongli Chemical Industry Co., Ltd., which is brown-black, with an iminodiacetonitrile content of 25%, quickly cool down to 45°C, feed sulfur dioxide gas until the pH of the reaction solution is 2.0, and cool down to 10-15°C. ℃, stirred and crystallized for 6.0 h, filtered, washed the solid with a small amount of water, and dried to obtain a white iminodiacetonitrile product 236.8, with an iminodiacetonitrile content of 98.9% and an impurity content of 0.15% in Compound I.
Embodiment 3
[0087] Take 1000 g of iminodiacetonitrile reaction solution produced by Xinjiang Ziguang Yongli Chemical Industry Co., Ltd., which is brown-black, with an iminodiacetonitrile content of 25%, quickly cool down to 45°C, feed sulfur dioxide gas until the reaction solution pH=4.0, and cool down to 10-15°C. ℃, stirred and crystallized for 6.0 h, filtered, washed the solid with a small amount of water, and dried to obtain 233.5 g of white iminodiacetonitrile product, the content of iminodiacetonitrile was 97.9%, and the impurity content of compound I was 0.52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com