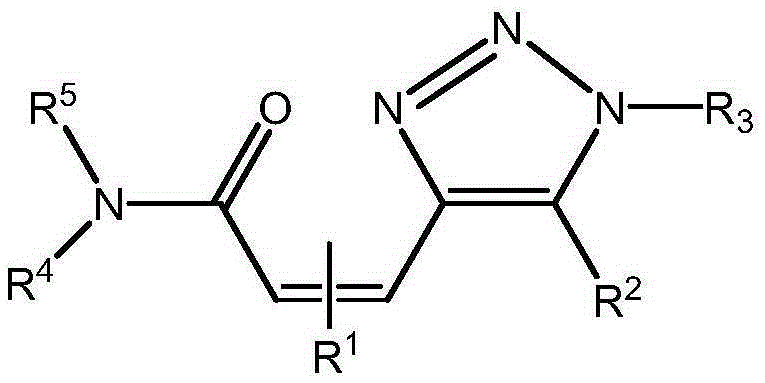
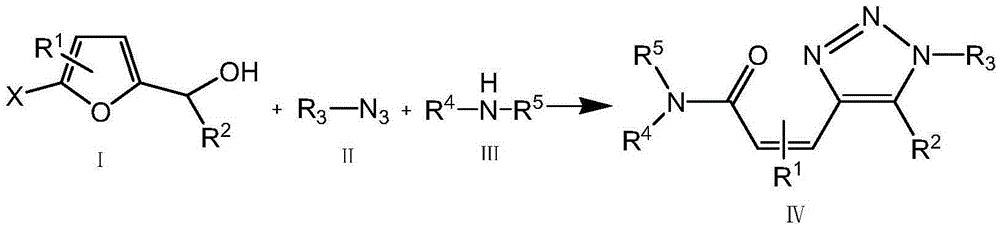Alkenyl amide triazole compound and synthetic method thereof
A technique for the synthesis of enamide triazoles, which is applied in organic chemistry, drug combinations, antineoplastic drugs, etc., can solve problems such as limitations and tedious multi-step synthesis, and achieve simple operation, high yield, and wide application range of substrates Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
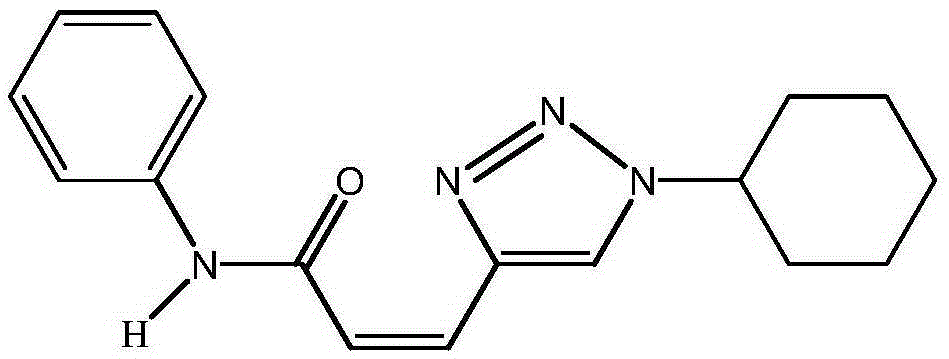
[0017] Taking the synthesis of (Z)-3-(1-cyclohexyl-1H-1,2,3-triazol-4-yl)-N-phenylacrylamide with the following structural formula as an example, the raw materials used and its synthesis method are as follows:
[0018]
[0019] Add 176mg (1.0mmol) of 5-bromo-2-furfuryl alcohol, 125mg (1.1mmol) of cyclohexyl azide, and 93mg (1.1mmol) of aniline into 5mL of dichloromethane, place in an ice-salt bath at -20°C, stir, and then Slowly add 0.21mL (1.5mmol) triethylamine and 1.1mL1mol / LTiCl dropwise 4 (1.1mmol) in dichloromethane, stirred at room temperature for 20 minutes, and the reaction mixture was washed with saturated NaHCO 3 Quenched, then diluted with 20mL ether, washed with saturated brine (2×5mL), extracted with ether (2×10mL), the organic phase was dried over anhydrous sodium sulfate and filtered, concentrated and washed with petroleum ether and ethyl acetate The mixed solution with a volume ratio of 1:1 was used as the eluent for column chromatography to obtain (Z)-3-(...
Embodiment 2
[0021] In Example 1, the triethylamine used was replaced with equimolar pyridine, and the other steps were the same as in Example 1 to obtain (Z)-3-(1-cyclohexyl-1H-1,2,3-triazole- 174 mg of 4-yl)-N-phenylacrylamide, the yield was 59%.
Embodiment 3
[0023] In Example 1, the triethylamine used was replaced by equimolar N,N-diisopropylethylamine, and the other steps were the same as in Example 1 to obtain (Z)-3-(1-cyclohexyl-1H- 1,2,3-triazol-4-yl)-N-phenylacrylamide 195 mg, the yield was 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com