New Trajenta crystal form and preparation method thereof
A technology of crystal form and ray powder diffraction, which is applied in the field of new crystal form of linagliptin, can solve problems such as inability to adapt to large-scale production, instability of crystal form A and B, etc., and achieve easy industrial preparation, good stability, crystal type stabilizing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 Preparation of new crystal form of linagliptin
[0037]
[0038](1) 8-[(3R)-3-Boc-amino-1-piperidinyl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-[( Add 6.1Kg of 4-methyl-2-quinazolinyl)methyl]-1H-purine-2,6-dione (compound II) into 62L of dichloromethane, then add 24.3kg of trifluoroacetic acid, and stir the reaction at room temperature 1.5h, add saturated sodium carbonate aqueous solution to adjust the pH value of the reaction solution to 6-8, separate the organic phase, evaporate the solvent under reduced pressure, and obtain an oil;
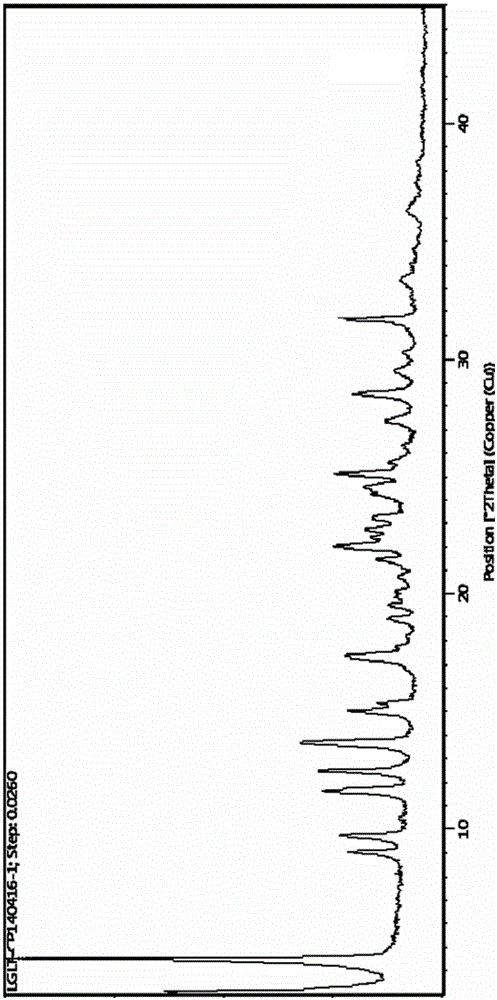
[0039] (2) Add 31.57L methanol to the oil obtained in step (1), heat to reflux to dissolve, add 1.33L purified water, keep stirring at 60°C until crystals precipitate, add 252L methyl tert-butyl ether, stir and cool down to 10-20°C ℃, filtered, and dried to obtain 3.85kg of white crystalline solid with a yield of 60.2%, HPLC purity of 99.94%, and moisture of 10.3%; after determination, its X-RPD spectrum is as follows: figu...
Embodiment 2
[0040] Embodiment 2 Preparation of new crystal form of linagliptin
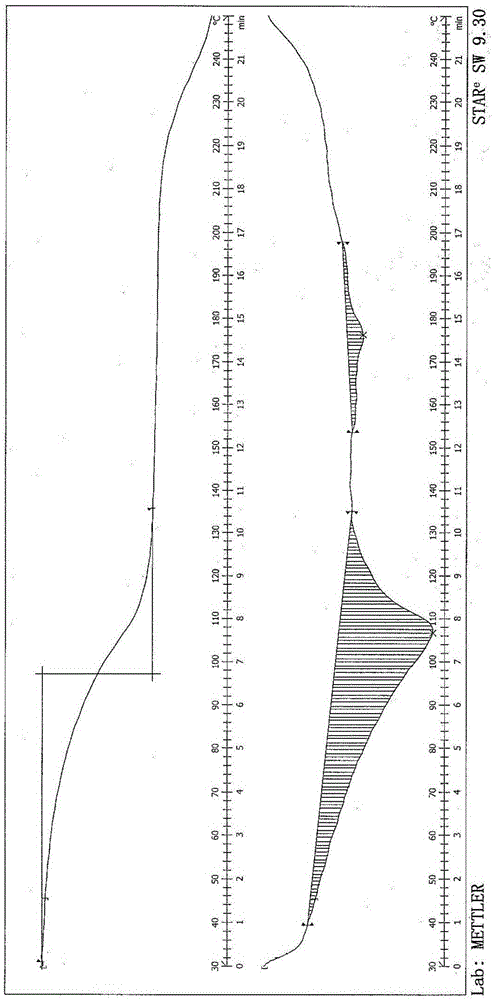
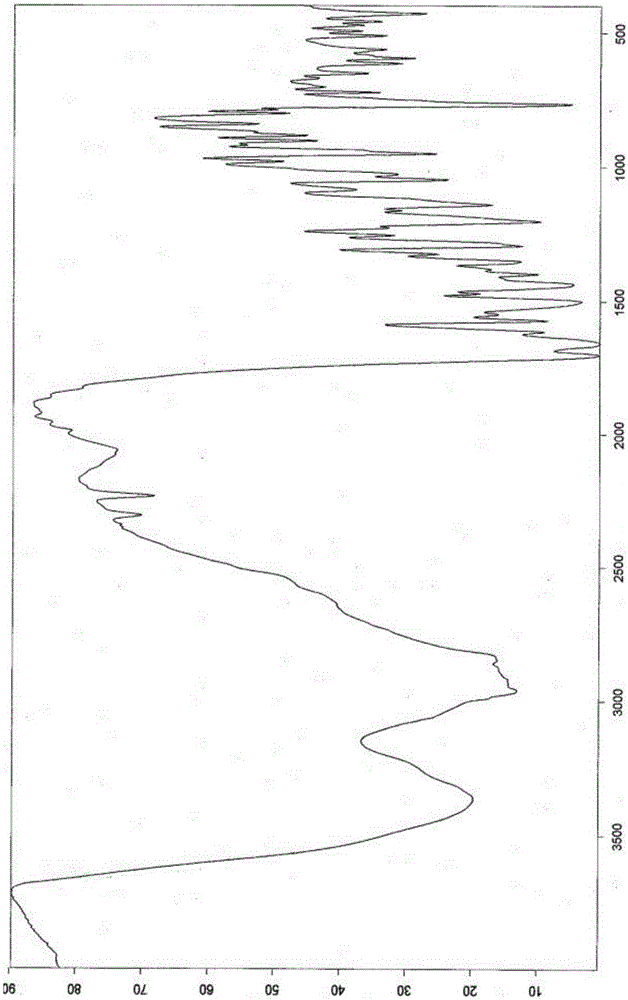
[0041] 8-[(3R)-3-Boc-amino-1-piperidinyl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-[(4-methyl Add 6 g of base-2-quinazolinyl)methyl]-1H-purine-2,6-dione into 48 mL of dichloromethane, add 18 g of trifluoroacetic acid, stir at room temperature for 1.5 h, add saturated aqueous sodium carbonate to adjust The pH value of the reaction solution was 6-8, the organic phase was separated, the solvent was distilled off under reduced pressure to obtain an oily substance; 90ml of ethanol was added, heated to reflux to dissolve, 6mL of purified water was added, and stirred at 55°C until crystals were precipitated, and 60ml of tertiary methyl Butyl ether was stirred and cooled to 25-30° C., filtered, and dried to obtain 3.45 g of white crystalline solid with a yield of 55.2%, a purity of 99.78%, and a water content of 10.1%. After determination, its X-RPD pattern and figure 1 Basically the same, its DSC-TGA spectrum and fi...
Embodiment 3
[0042] Embodiment 3 Preparation of new crystal form of linagliptin
[0043] 8-[(3R)-3-Boc-amino-1-piperidinyl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-[(4-methyl Add 6 g of base-2-quinazolinyl)methyl]-1H-purine-2,6-dione into 90 mL of dichloromethane, add 30 g of trifluoroacetic acid, stir at room temperature for 1.5 h, add saturated aqueous sodium carbonate to adjust The pH value of the reaction solution is 6-8, separate the organic phase, distill off the solvent under reduced pressure to obtain an oil; add 90ml of methanol, heat to reflux to dissolve, add 3mL of purified water, keep stirring at 60°C until crystals are precipitated, add 240ml of isopropyl acetate The ester was stirred and cooled to 20-30° C., filtered, and dried to obtain 3.72 g of a white crystalline solid with a yield of 59.4%, a purity of 99.74%, and a water content of 10.2%. After determination, its X-RPD pattern and figure 1 Basically the same, its DSC-TGA spectrum and figure 2 Basically the same, its i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com