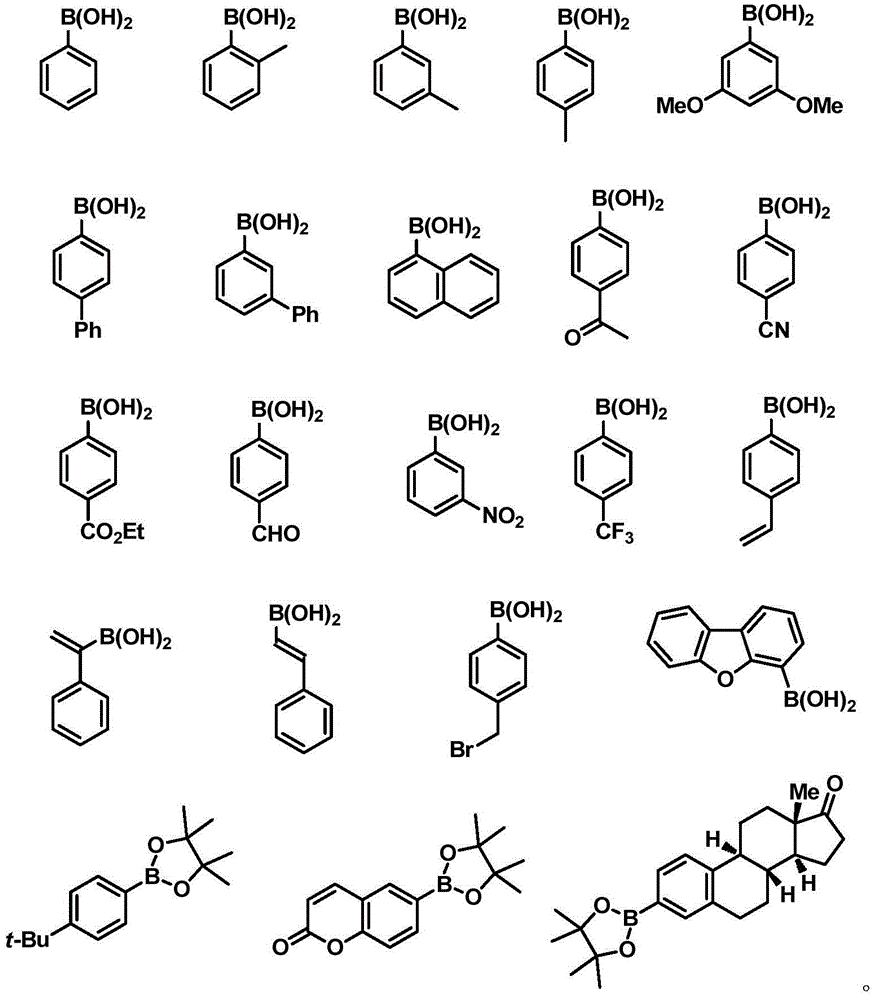
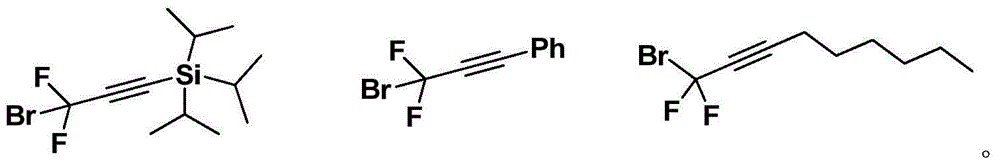
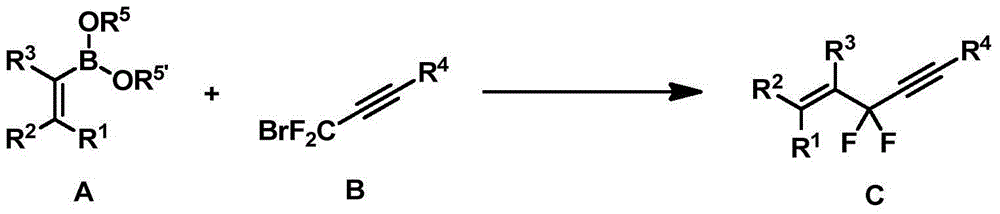Difluoropropargyl-containing compound, preparation method and applications thereof
A technology of difluoropropargyl and compound, applied in the preparation of organic compounds, steroids, organic silicon compounds, etc., can solve the problems of low reaction yield, limited substrate, lengthy reaction steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072]
[0073] To a 25 mL reaction tube, add 0.72 mmol phenylboronic acid, 2.8 mg (0.5 mol%) tris(dibenzylideneacetone) dipalladium, 5.6 mg (3 mol%) tris(o-methylphenyl)phosphine, potassium carbonate ( 1.8 mmol), inject 136 mg (0.6 mmol) of 1-triisopropylsilyl-3-bromo-3,3-difluoropropyne, 1,4-dioxane (3.0 mL). After stirring at 80°C for 24 hours, the yield was 91%, and the purity was greater than 98% as identified by hydrogen spectrum. 1 HNMR (400MHz, CDCl 3 )7.72-7.70(m,2H),7.45-7.41(m,3H),1.15-1.10(m,21H). 19 FNMR (376MHz, CDCl 3 )-74.3(s,2F). 13 CNMR (126MHz, CDCl 3 )136.2(t, J=27.9Hz), 130.6(t, J=1.9Hz), 128.4, 125.4(t, J=4.5Hz), 111.6(t, J=231.0Hz), 98.9(t, J=39.9 Hz), 92.5(t, J=4.9Hz), 18.5, 11.0.IR (thin film method) ν max 3440,2945,2868,2186,1742cm -1 .MS(EI):m / z(%)308(M+),265,115(100).HRMSCalculatedfor(theoretical value):308.1772; Found(measured value):308.1771.
Embodiment 2
[0075]
[0076] To a 25 mL reaction tube, add 0.72 mmol 2-methylphenylboronic acid, 2.8 mg (0.5 mol%) tris(dibenzylideneacetone) dipalladium, 5.6 mg (3 mol%) tris(o-methylphenyl)phosphine, Potassium carbonate (1.8 mmol), injection of 136 mg (0.6 mmol) 1-triisopropylsilyl-3-bromo-3,3-difluoropropyne, 1,4-dioxane (3.0 mL). After stirring at 80°C for 24 hours, the yield was 77%, and the purity was greater than 98% as identified by hydrogen spectrum. 1 HNMR (400MHz, CDCl 3 )δ7.68(d, J=7.9Hz, 1H), 7.34(t, J=7.5Hz, 1H), 7.28-7.20(m, 2H), 2.56(s, 3H), 1.16–1.05(m, 21H ). 19 FNMR (376MHz, CDCl 3 )δ-77.0(s,2F). 13 CNMR (126MHz, CDCl 3 )δ136.5(t, J=2.1Hz), 134.1(t, J=26.2Hz), 131.8, 130.4(t, J=1.5Hz), 125.63(t, J=7.4Hz), 125.57, 111.8(t , J=232.9Hz), 99.2(t, J=40.3Hz), 91.9(t, J=5.0Hz), 19.8, 18.5, 11.0.IR (thin film method) ν max 3416,2946,2868,2184,1463cm -1 .MS(EI):m / z(%)323(M + +H + ),322(M + ), 279.129(100).HRMS: Calculated (theoretical value): 322.1928; Found (measur...
Embodiment 3
[0078]
[0079] Into a 25 mL reaction tube, add 0.72 mmol 3-methylphenylboronic acid, 2.8 mg (0.5 mol%) tris(dibenzylideneacetone) dipalladium, 5.6 mg (3 mol%) tris(o-methylphenyl)phosphine, Potassium carbonate (1.8 mmol), injection of 136 mg (0.6 mmol) 1-triisopropylsilyl-3-bromo-3,3-difluoropropyne, 1,4-dioxane (3.0 mL). After stirring at 80°C for 24 hours, the yield was 85%, and the purity was greater than 98% as identified by hydrogen spectrum. 1 HNMR (400MHz, CDCl 3 )δ7.56–7.48(m,2H),7.32(t,J=7.6Hz,1H),7.28–7.26(m,1H),2.39(s,3H),1.14–1.08(m,21H). 19 FNMR (376MHz, CDCl 3 )δ-74.3(s,2F). 13 CNMR (126MHz, CDCl3) δ138.3, 136.1 (t, J = 27.6Hz), 131.4 (t, J = 1.8Hz), 128.3, 126.1 (t, J = 4.4Hz), 122.5 (t, J = 4.5Hz), 111.7(t, J=230.9Hz), 99.0(t, J=39.9Hz), 92.3(t, J=5.0Hz), 21.4, 18.5, 11.0.IR (thin film method) ν max 3387,2946,2868,2359,2182,1277cm -1 .MS(EI):m / z(%)322(M + ),279,129(100).HRMS: Calculated (theoretical value): 322.1928; Found (measured value): 322.1925....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com