Pumiloside preparation method
A kind of technology of scutellarin and isovinblastin lactam, applied in the preparation of sugar derivatives, chemical instruments and methods, organic chemistry and other directions, can solve the difficulty of separating and purifying scutellarin, problems such as low content, to achieve the effect of simple operation and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Study on the light irradiation stability of vinca lactam aqueous solution
[0045] Take an appropriate amount of vinca lactam, dissolve it with ultrapure water ultrasonically to prepare a solution of about 1.2mg / ml, filter, and divide the filtrate into 10ml vials, divide them into three groups, the first group is added to the solution Filled with nitrogen, the second group was filled with oxygen into the solution, the third group was air, plugged, capped, and sterilized (temperature: 121°C; time 20min). Due to the long light time, in order to eliminate the influence of microorganisms, it is preferable to perform high temperature sterilization on the vinca lactam solution. Samples were taken after the samples were placed at room temperature. The vinca lactam content in the samples before and after sterilization was detected by HPLC, and then the three groups of samples were stored at room temperature under natural light and dark place. After 7 days, samples were ...
Embodiment 2
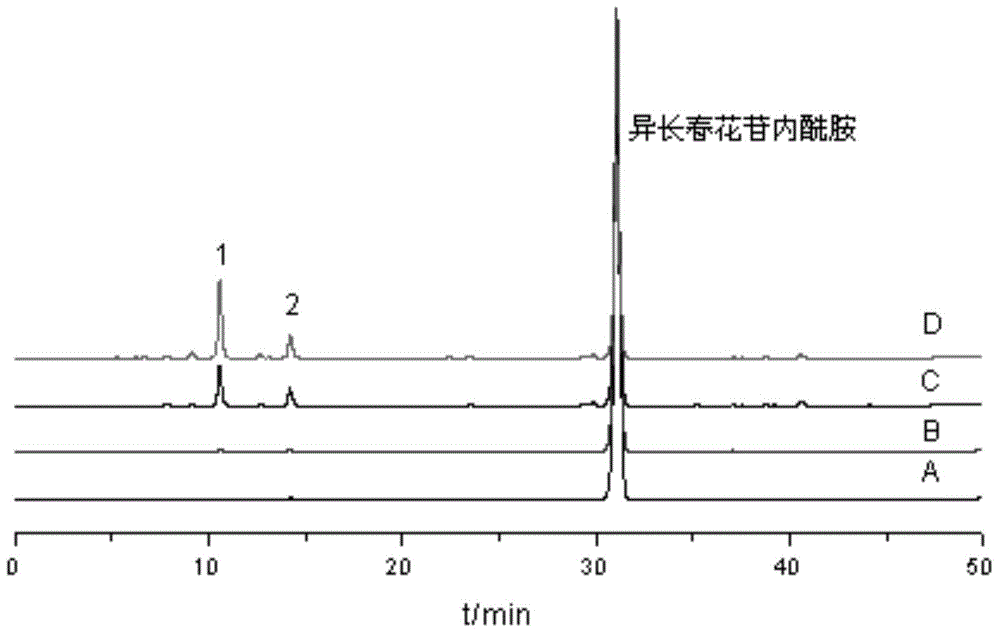
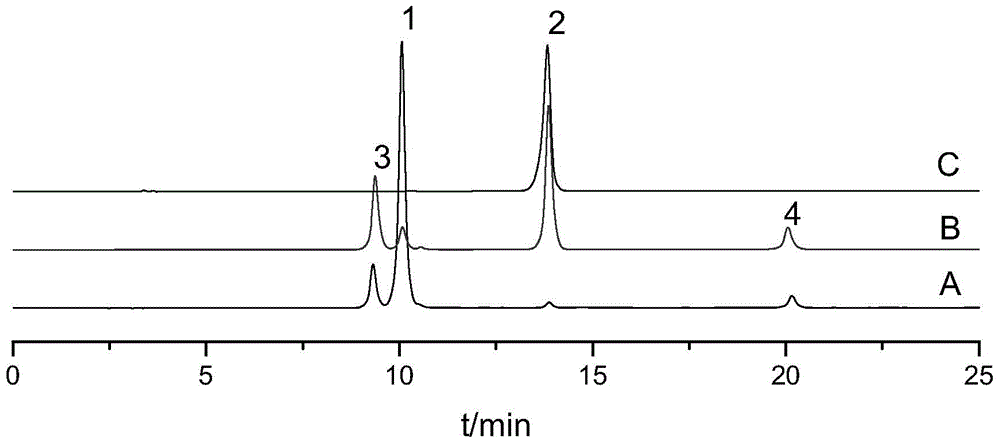
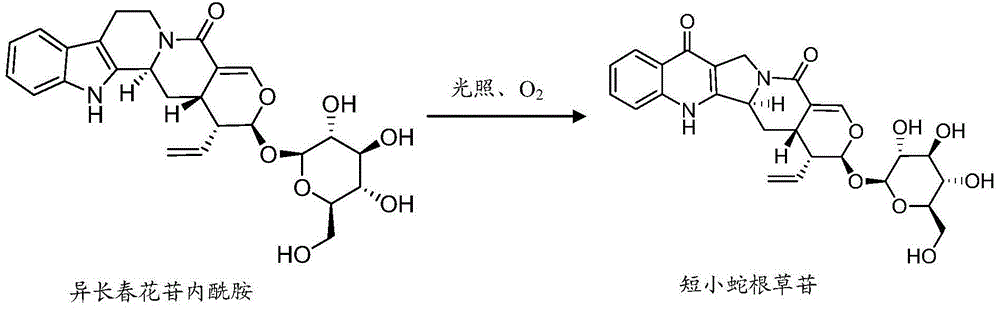
[0051] Weigh 0.7g of vinca lactam raw material, dissolve it with 500ml ultrapure water ultrasonically, filter, and divide the filtrate into 20ml vials, fill with oxygen, stopper, cap, and sterilize (temperature: 121℃; Time 20min). The sample is placed in natural light for 7 days, the reaction solution is passed through the HPD100 macroporous adsorption resin column, and eluted with water, 20% ethanol, 40% ethanol successively, and the 40% ethanol eluate is collected, concentrated and dried to obtain the main degradation Things ( figure 1 No. 1 and No. 2 chromatographic peaks) dry paste 262mg; the dry paste is dissolved in methanol and separated by liquid phase preparation, the mobile phase is acetonitrile-water (15:85), and the chromatographic peaks No. 1 and No. 2 are collected respectively The eluent. The separated degradation product No. 1 is unstable in the eluent, and it is still difficult to obtain an eluent containing a single chromatographic peak (see figure 2 ). In...
Embodiment 3
[0054] Weigh 0.7g of vinca lactam raw material, dissolve it with 500ml ultrapure water ultrasonically, filter, and divide the filtrate into 20ml vials, continue to infuse oxygen, sterilize at 100℃ for 60min, then place the sample in natural light After standing for 7 days, the reaction solution was passed through the HPD100 macroporous adsorption resin column, and then eluted with water, 20% ethanol, 40% ethanol in sequence, collected the 40% ethanol eluate, concentrated and dried to obtain 0.6 g of crude product, which was crystallized with methanol , To obtain white rod-shaped crystals with a yield of 70% and a purity of 90%. The structure of the obtained crude product was identified, and the result was the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com