Metal-phase carrier-loaded type catalyst, and preparation method and application thereof
A technology of carrier loading and metal phase, applied in the field of catalysis, can solve the problems of difficult preparation, poor thermal conductivity, poor stability, etc., and achieve the effects of simple preparation method, good thermal conductivity and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
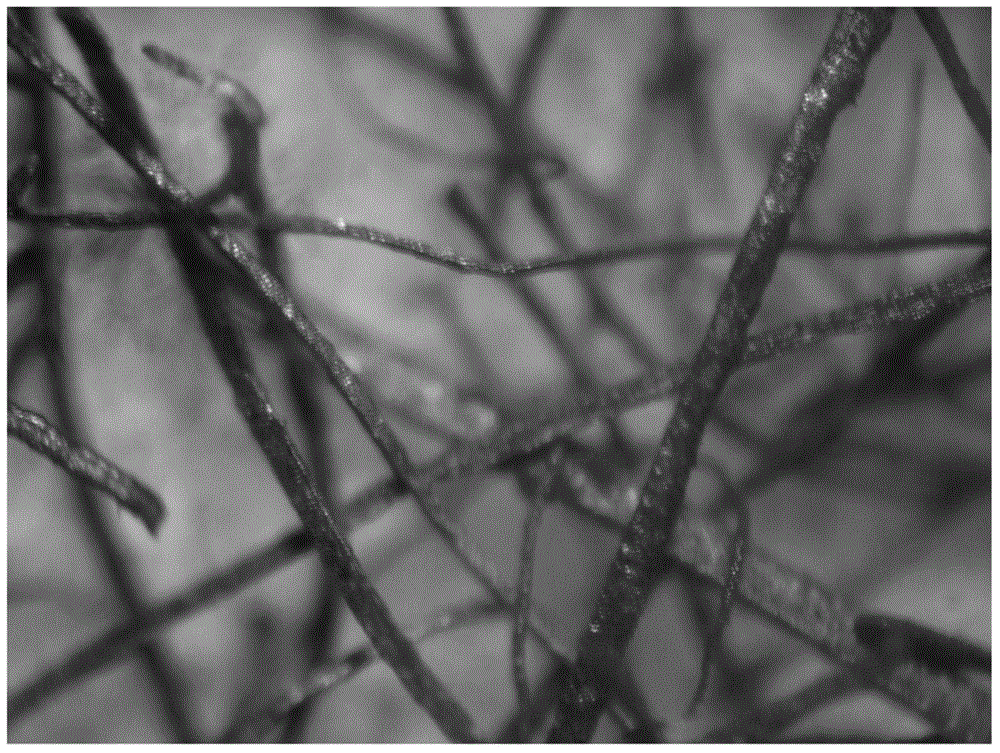
[0039] This example provides the preparation of a metal copper fiber carrier-supported palladium catalyst.
[0040]Weigh 2 grams of red copper fibers with a diameter of 30 microns and a length of 2-5 mm and place them in a 50 ml beaker, pipette 1 ml of palladium-containing 10 g / L palladium nitrate aqueous solution and drop them into the beaker to completely infiltrate the red copper metal fibers. After the galvanic replacement reaction between the surface of the red copper fiber and palladium nitrate occurred for 2 hours, it was dried and roasted in the air at 300°C for 2 hours to obtain a catalyst with a palladium weight content of 0.5%, expressed as Pd-0.5 / 30 -ZT-fiber-300.
[0041] Under the condition that other conditions remain unchanged, the diameter of copper metal fiber in this embodiment can be 8 microns, 80 microns and 120 microns, and the obtained catalysts are represented as Pd-0.5 / 8-ZT-fiber-300, Pd-0.5 / 80- ZT-fiber-300, Pd-0.5 / 120-ZT-fiber-300.
[0042] Under t...
Embodiment 2
[0044] This example provides the preparation of palladium catalysts supported by metal red copper fibers with different Pd precursors.
[0045] Weigh 2 grams of copper fiber with a diameter of 30 microns and a length of 2-5 mm and place it in a 50 ml beaker, pipette 1 ml of palladium-containing 10 g / L palladium acetate aqueous solution and drop it into the beaker to completely infiltrate the copper metal fiber. After the galvanic replacement reaction between the surface of the red copper fiber and palladium acetate occurred for 3 hours, it was dried and roasted in the air at 300°C for 2 hours to obtain a catalyst with a palladium weight content of 0.5%, expressed as Pd(ac)- 0.5 / 30-ZT-fiber-300.
[0046] The determination of element content by plasma inductively coupled atomic emission spectrometry shows that in the Pd(ac)-0.5 / 30-ZT-fiber-300 catalyst prepared in this example, the exact weight content of palladium is 0.48%, and the rest is copper. The elemental analysis result...
Embodiment 3
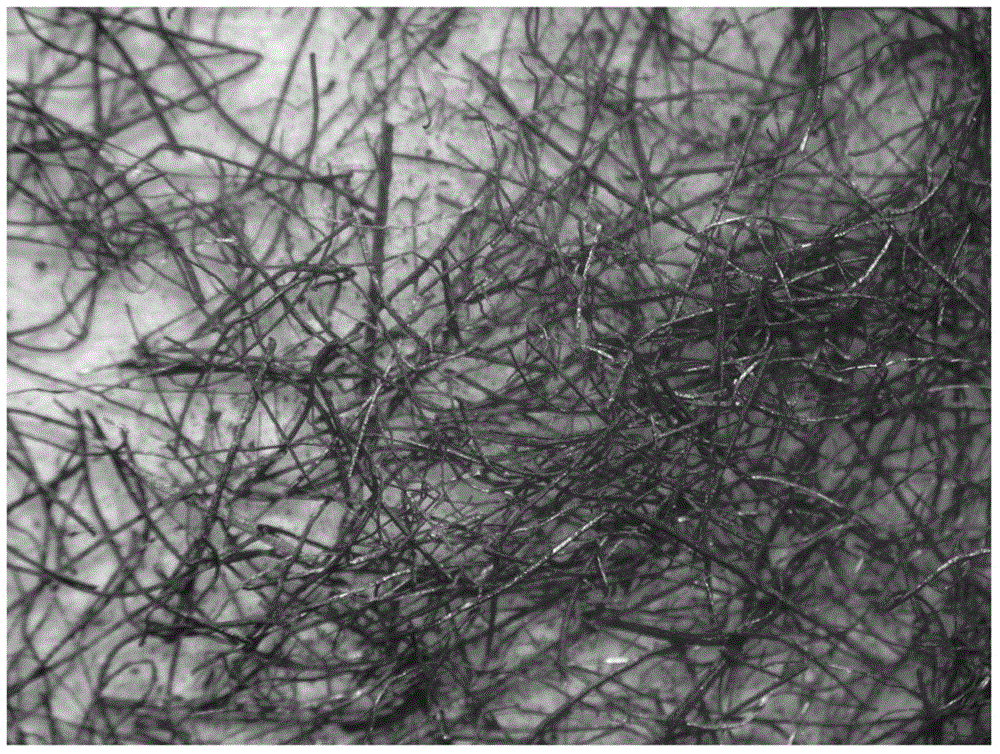
[0051] This example provides the preparation of different metal fiber carrier-supported palladium catalysts.
[0052] Weigh 2 grams of nickel fibers with a diameter of 8 microns and a length of 5 to 10 mm and place them in a 50-ml beaker, pipette 1 ml of palladium-containing 40 g / L palladium nitrate aqueous solution and drop them into the beaker to completely infiltrate the nickel metal fibers. After galvanic displacement reaction between the nickel fiber surface and palladium nitrate for 0.5 hours, dry and bake in the air at 500°C for 2 hours to obtain a catalyst with a palladium weight content of 2%, expressed as Pd-2 / 8 -Ni-fiber-500.
[0053] Under the condition that other conditions remain unchanged, the metal fibers used in this embodiment can be 90 micron brass or 30 micron aluminum fibers, and the catalysts obtained are respectively expressed as Pd-2 / 90-HT-fiber-500 and Pd-2 / 30-Al-fiber-500.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com