Guanidyl long chain gemini quaternary ammonium salt and preparation method thereof
A technology of gemini quaternary ammonium salt and diquaternary ammonium salt, which is applied in chemical instruments and methods, preparation of organic compounds, chemicals for biological control, etc., can solve complex reaction process, few action centers, poor hydrophilicity, etc. problem, to achieve the effect of simple reaction process, high surface activity and low foaming performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
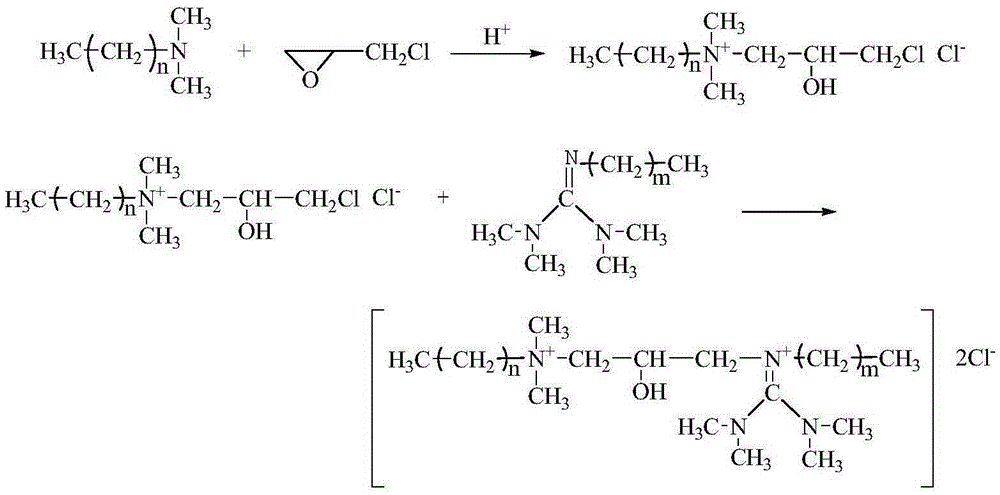
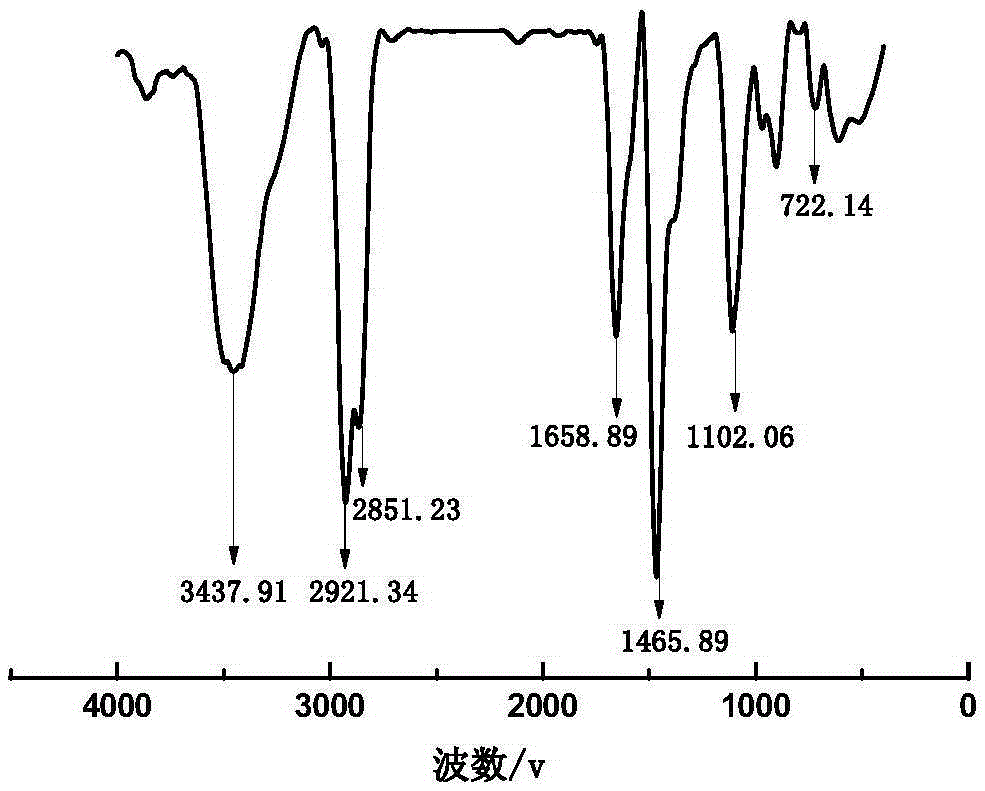
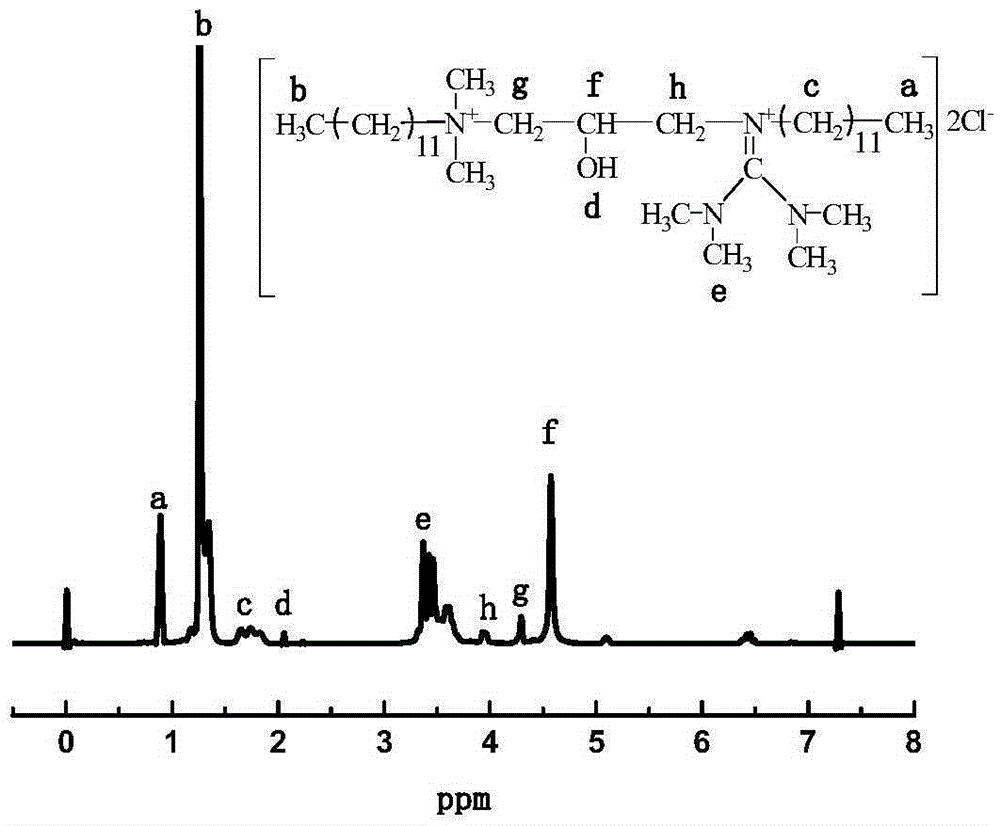
[0033] see figure 1 , 1) in the there-necked flask that NaCl is housed, drip the concentrated sulfuric acid (mass fraction is 98%) gradually, slightly heat, produce HCl gas, pass the HCl gas that produces into the concentrated sulfuric acid (mass fraction is 98%) and carry out drying , and then slowly poured into the ether solution containing dodecyldimethyl tertiary amine, stirring continuously at 25°C and 350r / min speed to obtain a white precipitate, which is dodecyldimethyl tertiary amine hydrochloride , After 2h, the reaction ended. Wash with ether several times to obtain pure dodecyl dimethyl tertiary amine hydrochloride, dry at 20°C for 6 hours, and set aside.
[0034] 2) Add 7.497g (0.03mol) dodecyldimethyl tertiary amine hydrochloride and 0.3195g (0.0015mol) dodecyldimethyl tertiary amine to a three-necked flask containing 25mL of acetone, at 30°C , stirring at 400r / min for 45min; raise the temperature to 50°C, at 500r / min, add 2.275g (0.03mol) of epichlorohydrin (EC...
Embodiment 2
[0037] 1) with embodiment 1.
[0038] 2) Add 7.497g (0.03mol) dodecyldimethyl tertiary amine hydrochloride and 0.0639g (0.0003mol) dodecyldimethyl tertiary amine to a three-necked flask filled with 30mL acetone, at 30°C, Stir at 400r / min for 45min; raise the temperature to 60°C, at 450r / min, add 2.275g (0.03mol) of epichlorohydrin (ECH) dropwise within 60min, stop the reaction after 4h; distill under reduced pressure to remove Remove excess solvent and reactants, and perform multiple recrystallizations in acetone to obtain 2-hydroxypropyl-3-chloro-dimethyl dodecyl ammonium chloride.
[0039] 3) Add 10.26g (0.03mol) 2-hydroxypropyl-3-chloro-dimethyldodecyl ammonium chloride to a three-necked flask filled with 30mL of ethanol, and stir at 30°C and 400r / min After 10 minutes, the temperature was raised to 60°C, and 3.999 g (0.031 mol) of 1,1,2,3,3-pentamethylguanidine was gradually added dropwise at 450 r / min, and the addition was completed after 30 minutes. Stop the reaction af...
Embodiment 3
[0041] 1) with embodiment 1
[0042] 2) Add 7.497g (0.03mol) dodecyldimethyl tertiary amine hydrochloride and 0.639g (0.003mol) dodecyldimethyl tertiary amine to a three-necked flask containing 20mL of acetone, at 30°C, Stir at 400r / min for 45min; raise the temperature to 60°C, at 450r / min, add 3.2375g (0.035mol) epichlorohydrin (ECH) dropwise within 60min, stop the reaction after 5h; distill under reduced pressure to remove Remove excess solvent and reactants, and perform multiple recrystallizations in acetone to obtain 2-hydroxypropyl-3-chloro-dimethyl dodecyl ammonium chloride.
[0043] 3) Add 10.26g (0.03mol) of 2-hydroxypropyl-3-chloro-dimethyl dodecyl ammonium chloride to a three-necked flask containing 20mL of ethanol solution, and stir fully at 30°C and 400r / min After 10 minutes, the temperature was raised to 70°C, and 4.128 g (0.032 mol) of 1,1,2,3,3-pentamethylguanidine was gradually added dropwise at 450 r / min, and the addition was completed after 30 minutes. After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com