Ph sensor made of near-infrared light-emitting ru complex
A technology of binuclear ruthenium complexes and complexes, applied in the field of pH sensing, to achieve the effects of stable structure, easy operation, and guaranteed accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
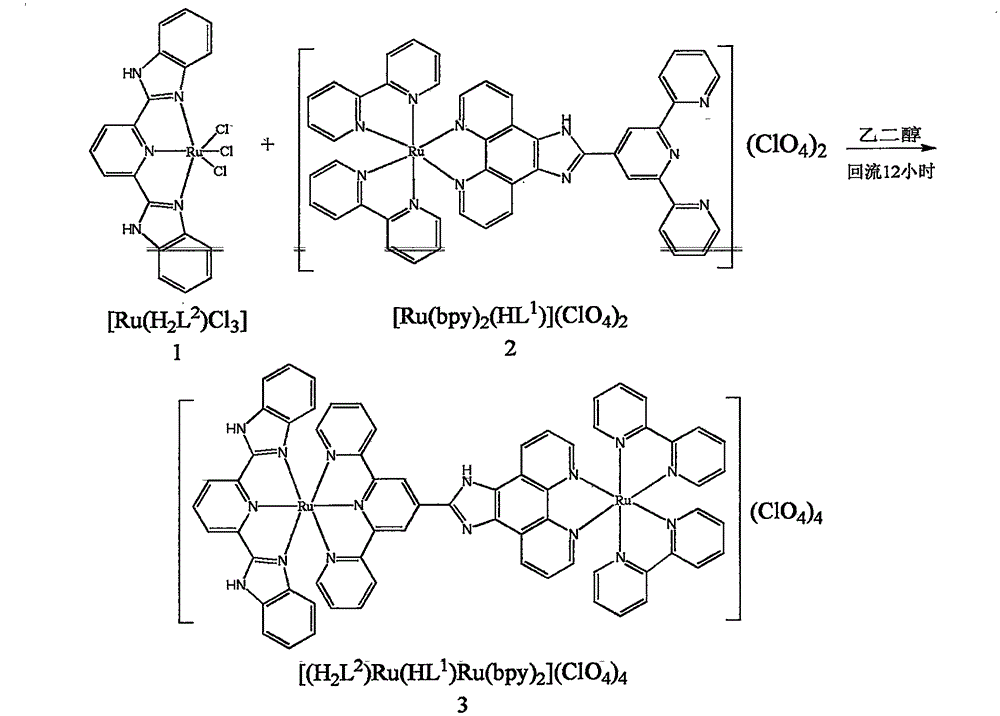
[0025] Embodiment 1: Complex [(bpy) 2 Ru(HL 1 )Ru(H 2 L 2 )] (ClO4 ) 4 The preparation steps:
[0026] (1) Ligand H 2 L 2 Synthesized according to literature [Addison, A.W.; Burke, P.J. Heterocycl. Chem. 1981, 18, 803-805.].
[0027] (2)[Ru(H 2 L 2 ) Cl 3 ] is RuCl 3 ·3H 2 O and ligand H 2 L 2 According to the 1:1 molar ratio in ethanol in the reflux heating system.
[0028] (3) Ligand HL 1 and [Ru(bpy) 2 HL 1 ](ClO 4 ) 2 According to literature [Zheng, Z.B.; Duan, Z.M.; Zhang, J.M.; Wang, K.Z. ZhiWang, HighlySensitiveandSelectiveDifunctionalRuthenium (II) ComplexBasedChemosensorforDihydrogenPhosphateAnionandFerrousCation, Inorg.Chem.2013, 52, 2306-2316] synthesis.
[0029] (4) Complexes [(bpy) 2 Ru(HL 1 )Ru(H 2 L 2 )] (ClO 4 ) 4 Preparation, weigh [Ru(H 2 L 2 ) Cl 3 [Ru(bpy) 2 HL 1 ](ClO 4 ) 2 (0.161g, 0.15mmol), the mixed solution was heated and refluxed at 180°C for 12 hours under the protection of nitrogen. After the reaction, the solution...
Embodiment 2
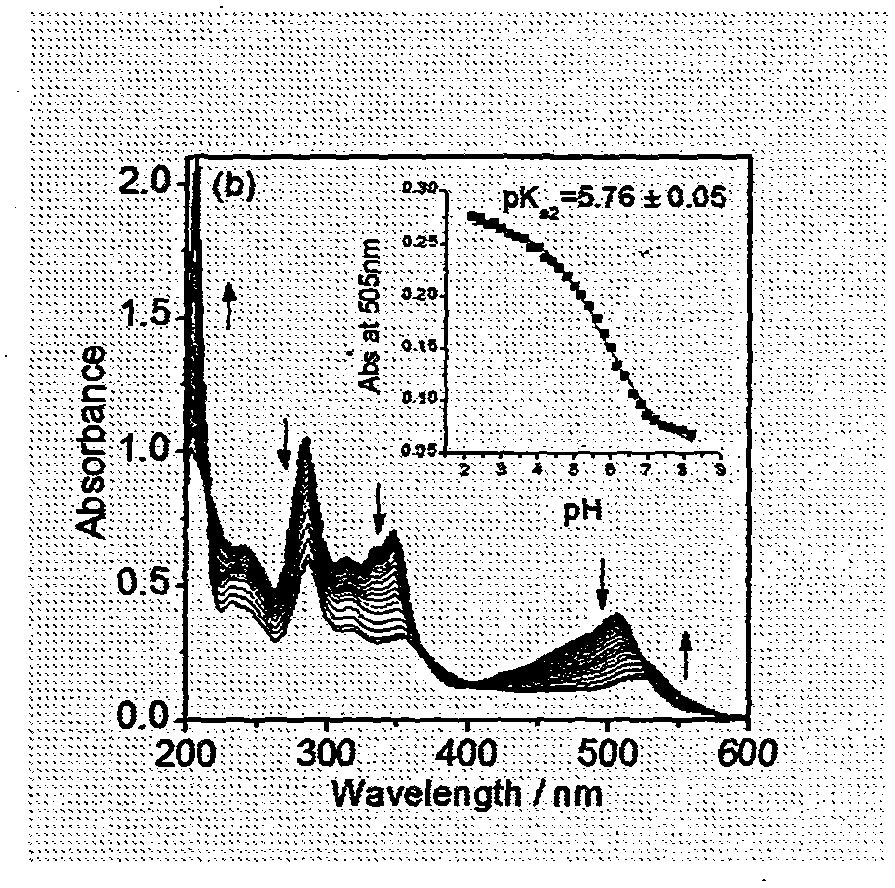
[0032] Embodiment 2: The mensuration of ultraviolet-visible absorption spectrum and emission spectrum and the drawing of working curve when different pH
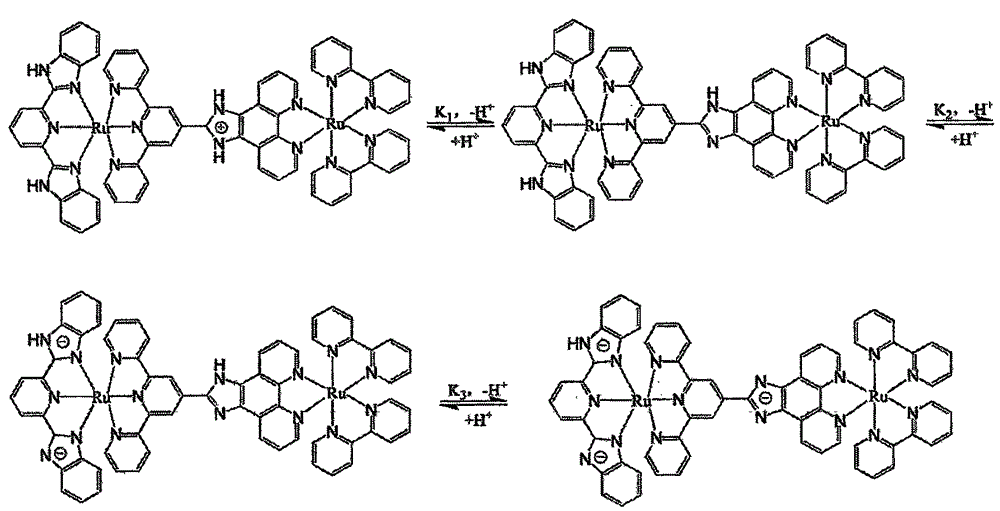
[0033] The acid-base titration of the complex was carried out in Britton-Robinson (abbreviated as BR) buffer solution. BR buffer solution is a mixture of 0.04M glacial acetic acid, 0.04M boric acid and 0.1M sodium chloride. Sodium chloride is used to maintain the ionic strength of the system, thereby reducing the influence of the external environment on the test. Prepare 40ml 3.75×10 -6 The mol / L complex solution to be tested was divided into two parts, and the pH of one part was adjusted with concentrated sulfuric acid, and the ultraviolet-visible absorption and emission spectra of Ph=0.2-2.0 were measured. Another part is adjusted pH with concentrated sodium hydroxide solution, measures the ultraviolet-visible absorption of pH=2.0-7.0 and emission spectrum (excitation wavelength λ ex = 505nm). Measure a data every 0.2 ...
Embodiment 3
[0040] Embodiment 3: the mensuration of unknown water sample pH
[0041] Take 23ml of unknown water sample, add sodium chloride to it to a concentration of 0.1M, and keep the ionic strength consistent with that of BR buffer. Take 3ml of water sample with sodium perchloride as reference solution, add quantitative complex to the remaining 20ml of water sample to make the concentration 3.75×10 -6 mol / L, which is consistent with the concentration in the acid-base titration of the complex. Measure the UV absorption and emission spectra of water samples.
[0042] The quantum efficiency of luminescence is obtained by ruthenium terpyridine [Ru(bpy) 3 ] 2+ as a standard (Φstd =0.028), the measured concentration is 1.0×10 -6 mol / L [Ru(bpy) 3 ] 2+ The UV-visible absorption spectrum and emission spectrum of the aqueous solution, read the absorbance A at 450nm of the UV-visible absorption spectrum std and the integrated intensity I of the emission spectrum std , according to formul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com