Ulipristal acetate crystal G type substance as well as preparation method, composition and applications thereof
A technology of ulipristal acetate and composition, applied in directions such as drug combination, steroids, pharmaceutical formulations, etc., can solve the problem of not finding ulipristal acetate crystal form patents or literature reports, etc., and achieve the advantages of stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Preparation method 1 of ulipristal acetate crystal type G substance sample:
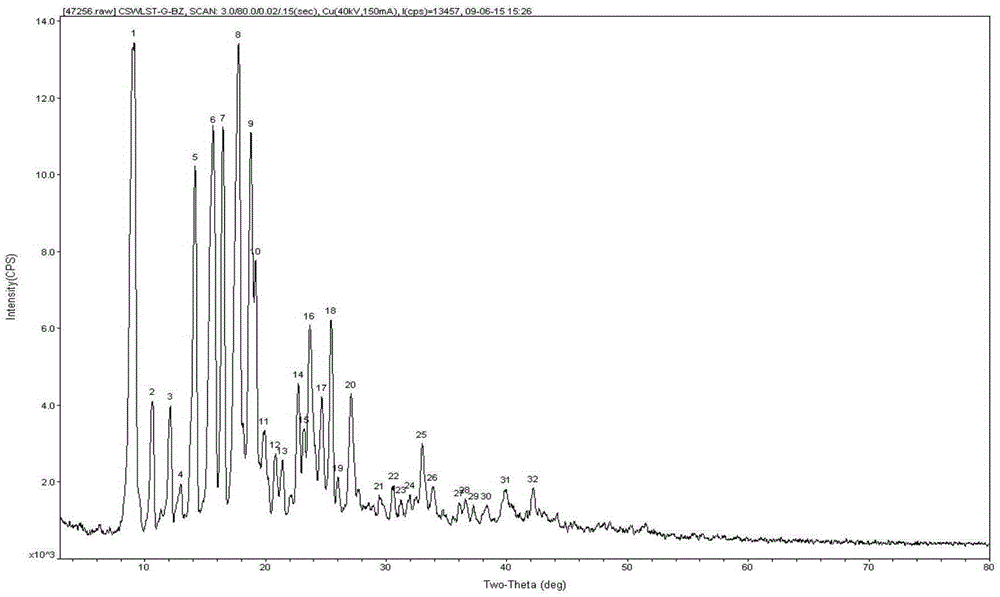
[0071] Use acetone solvent to completely dissolve the ulipristal acetate sample at room temperature at 20°C, stir at 30°C for 24 hours to obtain the acetone solvate of ulipristal acetate, and place the solvate at 130°C Put it under 1 hour to remove solvent and prepare to obtain ulipristal acetate solid sample, carry out powder X-ray diffraction analysis to it, its diffraction picture Spectrum and figure 1 Consistent, indicating that the obtained sample is ulipristal acetate crystal G type solid matter.
[0072] Preparation method 2 of ulipristal acetate crystal type G substance sample:
[0073] Use ethanol solvent to completely dissolve the ulipristal acetate sample at room temperature at 20°C, stir at 30°C for 24 hours to obtain the ethanol solvate of ulipristal acetate, and place the solvate at 135°C Put it under 1 hour to remove solvent and prepare to obtain ulipristal acetate solid ...
Embodiment 2
[0079] Ulipristal acetate crystal G type solid substance stability characteristics:
[0080] High temperature test: put the crystal form sample in an open clean watch glass, place it at 60°C for 10 days, and take samples on the 0th day, the 5th day and the 10th day. The samples obtained from the above sampling points were subjected to powder X-ray diffraction analysis, and the diffraction picture spectrum mean figure 1 Consistent, showing that ulipristal acetate crystal G type solid matter is stable under the high temperature influence factor test.
[0081] High-humidity test: Put the crystal sample in an open clean watch glass, place it at 25°C for 10 days at a relative humidity of 90%±5%, and take samples on the 0th day, the 5th day and the 10th day. The samples obtained from the above sampling points were subjected to powder X-ray diffraction analysis, and the diffraction picture spectrum mean figure 1 Consistent, showing that ulipristal acetate crystal G type s...
Embodiment 3
[0085] The preparation method 1 (tablet) of combination pharmaceutical preparation:
[0086] A method for preparing a combination drug tablet, which is characterized in that the pure product of ulipristal acetate crystal G type material, or the mixed crystal solid material containing crystal G type in any proportion is used as the raw material drug of the combination drug, and several excipients are used. As an auxiliary material component for the preparation of combined drug tablets, a tablet sample with a drug content of 10-500 mg per tablet is prepared according to a certain ratio. Table 2 Give the tablet formulation proportions:
[0087] surface 2 preparation formulas of ulipristal acetate combination medicine tablet
[0088]
[0089]
[0090] The method for preparing the pure product of ulipristal acetate crystal G type substance or the mixed crystal raw material drug containing crystal G type in any proportion into a tablet preparation is as follows: mix severa...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap